Table 1.
Mukaiyama Aldol Reactions of 2-Siloxyindoles and Isatinsa
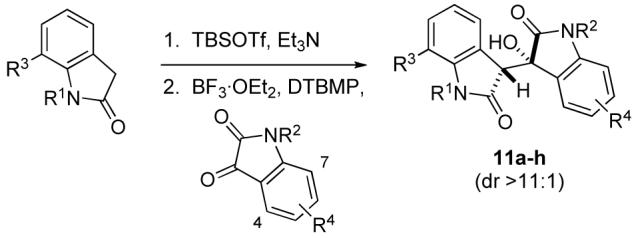 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R3 | R2 | R4 | 11 | Yield (%)b |
| 1 | SEM | H | Me | 4-vinyl | 11a | 77 |
| 2 | Me | MeO | SEM | H | 11b | 87 |
| 3 | Me | MeO | Bn | H | 11c | 80 |
| 4 | SEM | H | Bn | H | 11d | 76 |
| 5 | Bn | H | SEM | 7-F | 11e | 83 |
| 6 | SEM | H | Bn | 6-phenyl | 11f | 84 |
| 7 | SEM | H | Bn | 5-(3-pyridyl) | 11g | 60 |
| 8 | Me | MeO | Me | 4-vinyl | 11h | 52c |
Conditions: Oxindole (1.0 equiv), isatin (1.0 equiv), BF3·Et2O (2.0 equiv), DTBMP (2.5 equiv), –78 °C to –50 °C.
Yield over two steps of the aldol adduct after purification by silica gel flash column chromatography.
The starting oxindole (15–20%) was recovered.
