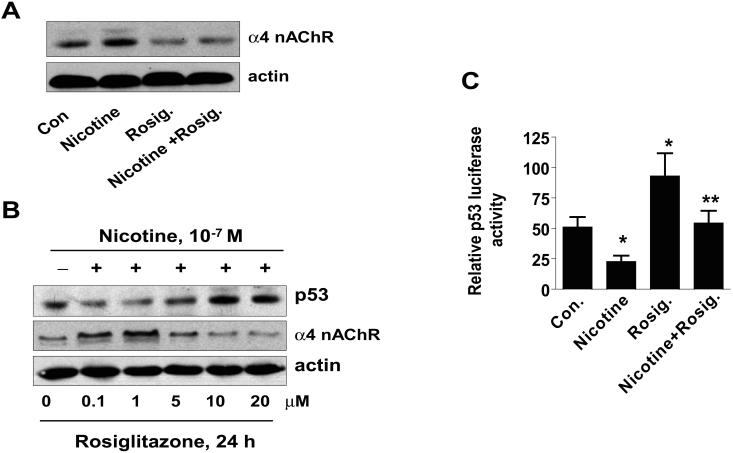
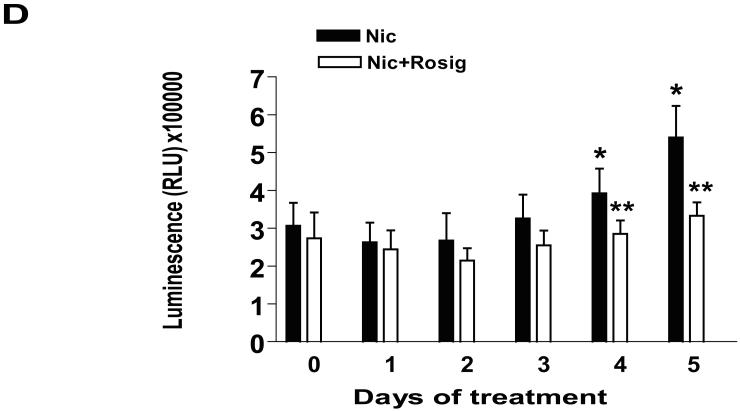
Figure 5. Rosiglitazone overcomes the effect of nicotine on expression of p53 and α4 nAChR.
A, Cellular protein (20 μg) was isolated from H1838 cells treated with Rosig. (10 μM) or nicotine (0.1 μM) for 24 h, or Rosig. for 2 h before exposure of the cells to nicotine for an additional 24 h, followed by Western blot analysis for α4 nAChR protein. Blots were also incubated with an anti-actin antibody for normalization purposes. Con, indicates untreated control cells. B, Cellular protein was isolated from H1838 cells treated with increasing concentrations of rosiglitazone (Rosig.) for 24 h in the presence or absence of nicotine (0.1 M) followed by Western blot analysis for p53 and α4 nAChR proteins using an anti-p53 or α4 nAChR antibodies. Actin served as internal control for normalization purposes. C, H1838 cells were transfected with a mixture of inducible p53-responsive firefly luciferase construct and constitutively expressing Renilla luciferase construct (40:1, 0.1 μg/μl) for 24 h, then treated rosiglitazone (Rosig., 10 μM) for 2 h before exposure of the cells to nicotine for an additional 24 h. The ratio of firefly luciferase to renilla luciferase activity was quantified as described in Material and Methods. The bars represent the mean ± SD of at least four independent experiments for each condition. * indicates significant increase of activity as compared to control untreated cells. ** indicates significance of combination treatment as compared with rosiglitazone (Rosig.) or nicotine alone (P < 0.05). Con, indicates untreated control cells. D, H1838 cells were cultured with rosiglitazone (Rosig., 10 μM) alone, nicotine (Nic., 100 μM) alone or Rosig. (10 μM) plus Nic. (100 μM) for 5 days. Afterward, the luminescence of viable cells was detected using Cell Titer-Glo Luminescent Cell Viability Assay Kit according to the protocol of the manufacturer. All data are depicted as mean ± SD. * indicates significant difference from day zero in Rosig. or nicotine alone. ** indicates significant difference from day zero in combination treatment (Rosig. plus nicotine) (P<0.05).