Figure 3.
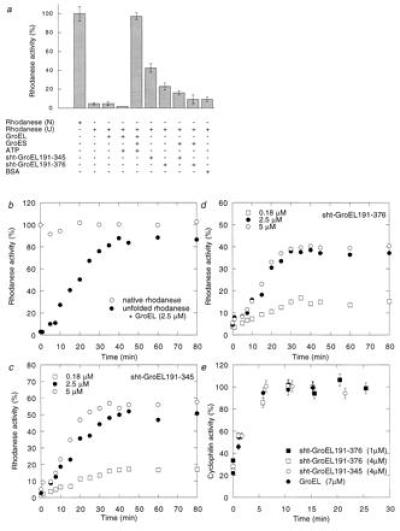
Refolding of rhodanese and cyclophilin A in the presence of sht-GroEL191-345 and sht-GroEL191-376. (a) Relative enzymatic activity of rhodanese (0.1 μM) after refolding in the presence (+) or absence (−) of GroEL (2.5 μM monomer), GroES (2.5 μM monomer), ATP (2 mM), sht-GroEL191-345 (2.5 μM), sht-GroEL191-376 (2.5 μM), or bovine serum albumin (45 μg/ml), from 8 M urea (U). One-hundred percent activity was obtained with native rhodanese (N). (b) Refolding kinetics of rhodanese in presence of GroEL, GroES, and ATP. The final concentrations are the same as in a. One-hundred percent activity was obtained with native rhodanese. (c and d) Refolding kinetics of rhodanese in the presence of 0.18 μM, 2.5 μM, or 5 μM sht-GroEL191-345 and sht-GroEL191-376, respectively. (e) Refolding of 1 μM cyclophilin A in the presence of 7 μM GroEL (monomer), 4 μM sht-GroEL191-345, 4 μM sht-GroEL191-376, or 1 μM sht-GroEL191-376. One-hundred percent activity was obtained with native cyclophilin A. Standard error bars are shown. The 30% spontaneous refolding of cyclophilin was complete in the dead time of the experiment.
