Abstract
The class-specific transcription factors Knot and Cut act during dendrite arbor development to define the characteristic dendrite branching pattern of the Drosophila class IV dendritic arborisation sensory neurons. Knot mediates dendrite arbor outgrowth and branching via a microtubule-based program that includes upregulation of the microtubule severing protein Spastin. On the other hand, Cut promotes dendrite arbor outgrowth and branching through a filamentous-actin based program and additionally promotes filopodia formation. We discuss how differential regulation of the activity of the Rac1 small GTPase by Knot and Cut may underlie some of the different roles these transcription factors play during class-specific dendrite arbor morphogenesis.
Key words: dendrite, morphology, knot, cut, rac1, filopodia, branch, neuron, drosophila, spastin
Dendrites are the chief site of signal input into a neuron, and the elaborate shape of dendrite arbors influences their ability to process these separate signals into meaningful computation.1 Given the essential role of the dendrite arbor for correct neuron function, an important question in developmental neuroscience is that of how the characteristic arbor shapes of different neuron classes are established.
A series of recent studies using the dendritic arborization (da) sensory neurons of the Drosophila larva has shown that neuron class-specific dendrite arbor shape is controlled by class-specific codes of transcription factors acting in post-mitotic, differentiating neurons.2–8 Mature dendrite arbor shape is the outcome of a succession of complex developmental processes. Hence one possibility is that the function of these transcription factors is to modulate basic dendritic developmental programs common to all neuron types in a class-specific manner.
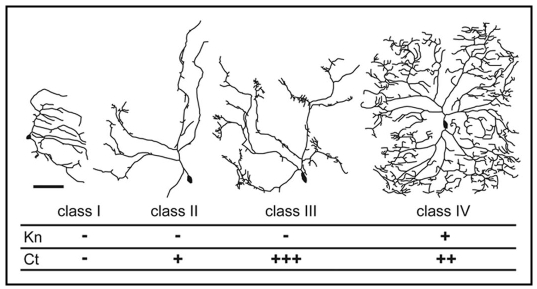
There are four classes of da neurons (I–IV), each with characteristic dendrite arbor morphology (Fig. 1).9 We recently demonstrated that a transcription factor code consisting of class IV-specific expression of the zinc finger, helix-loop-helix protein Knot and an ‘intermediate’ level of the homeodomian protein Cut, defines the characteristic dendrite arbor shape of this neuron class.5 We found that Cut promotes outgrowth that is microtubule deficient and filamentous (F)-actin rich. On the other hand, Knot promotes extensions of the dendrite arbor entirely positive for the Drosophila MAP1B homologue Futsch, which co-localizes with microtubules.10,11 Hence Knot mediates dendrite outgrowth positive for the microtubule cytoskeleton.
Figure 1.
Transcription Factor Codes that Specify Neuron Class-Specific da Dendrite Arbor Shape. Tracings to illustrate the dendrite arbor morphology of the four classes of da neuron: class I ddaE, class II ddaB, class III ddaF and class IV ddaC at wandering third instar stage. Anterior is to the left, dorsal is up, the scale bar is 75 µm. The expression of the different class-specific transcription factors is shown. For Cut: − no protein expression, + low, ++ intermediate, +++ high. This figure is reproduced with modifications from Jinushi-Nakao et al. (2007).
In addition to having dissimilar effects on the dendrite cytoskeleton, we have also found that Knot and Cut interact differently with the Rho family small GTPase Rac1, an important regulator of F-actin polymerization.12 When Rac1 was co-expressed with Cut, it enhanced the ability of Cut to promote filopodia formation.5 As a normal function of Cut is promoting filopodia formation, it seems likely that an interaction with Rac1 is part of this process (Fig. 2). On the other hand, when Rac1 was co-expressed with Knot, together they caused a large increase in dendrite branch formation. It has previously been shown that Rac1 is required for class IV neuron dendrite branching.13 Could an interaction between Knot and Rac1 be important for the formation of some class IV branches?
Figure 2.
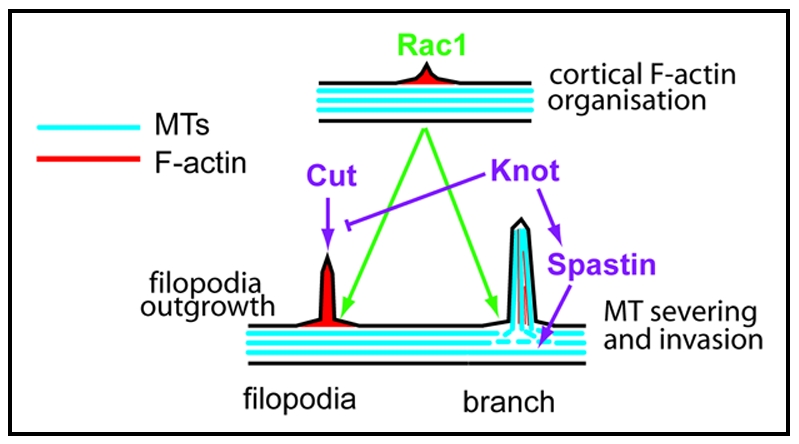
Differential Modulation of Rac1 activity by Cut and Knot May lead to Filopodia or Branch Formation. Rac1 mediates local F-actin rearrangement at the dendrite cortex. Rac1 activity may provide a local site (green arrows) for the action of factors controlled by either the Knot or Cut mediated pathways (purple arrows). Cut controls a pathway leading to filopodia formation from this local site. Knot upregulates Spastin expression. Spastin then causes localized microtubule (MT) severing at the region where Rac1 has promoted protrusion formation. Rac1 at the cortex mediates microtubule capture. Invasion of microtubules into this protrusion causes its stabilization and results in dendrite branch formation.
A key step in branch and neurite outgrowth is microtubule invasion along an F-actin core.15 Knot mediates microtubule-based dendrite arbor outgrowth and could function at this microtubule invasion step. Knot upregulates expression of the AAA (ATPases Associated with diverse cellular Activities) family microtubule severing protein Spastin.5 Spastin promotes branching in both the axonal and dendritic compartments by providing short microtubules for invasion at putative branch-points.14 During mammalian cell polarization and migration, Rac1 marks localized sites of the cortical actin cytoskeleton to which the Rac1 effector IQGAP1 is targeted. IQGAP1 then captures microtubules via interaction with CLIP-170, a protein that is localized at the plus ends of microtubles.16 It is tempting to speculate that a similar process may occur during dendrite branching; Rac1 could mark a site where the short microtubules produced by Spastin can invade and promote branch formation (Fig. 2).
Different interactions with Rac1 could be an important part of mechanisms by which Knot and Cut have different roles in dendrite formation. To further elucidate how Knot and Cut function, it is now important to identify transcriptional targets of Knot and Cut activity, and elucidate how they modify the cytoskeleton. The strength of Drosophila genetics, coupled with emerging powerful molecular biological techniques to identify transcriptional targets, could greatly aid in identifying core components of dendrite development that are modulated in a class-specific manner.
Acknowledgements
I thank Jeffrey Robens, Andrew Liu and Niall Murphy for critical reading and RIKEN for funding.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6395
References
- 1.London M, Hausser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- 2.Crozatier M, Vincent A. Control of multidendritic neuron differentiation in Drosophila: the role of Collier. Dev Biol. 2008;315:232–242. doi: 10.1016/j.ydbio.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 3.Grueber WB, Jan LY, Jan YN. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003;112:805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 4.Hattori Y, Sugimura K, Uemura T. Selective expression of Knot/Collier, a transcriptional regulator of the EBF/Olf-1 family, endows the Drosophila sensory system with neuronal class-specific elaborated dendritic patterns. Genes Cells. 2007;12:1011–1022. doi: 10.1111/j.1365-2443.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- 5.Jinushi-Nakao S, Arvind R, Amikura R, Kinameri E, Liu AW, Moore AW. Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron. 2007;56:963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Wang F, Menut L, Gao FB. BTB/POZ-zinc finger protein abrupt suppresses dendritic branching in a neuronal subtype-specific and dosage-dependent manner. Neuron. 2004;43:823–834. doi: 10.1016/j.neuron.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Moore AW, Jan LY, Jan YN. hamlet, a binary genetic switch between single- and multiple-dendrite neuron morphology. Science. 2002;297:1355–1358. doi: 10.1126/science.1072387. [DOI] [PubMed] [Google Scholar]
- 8.Sugimura K, Satoh D, Estes P, Crews S, Uemura T. Development of morphological diversity of dendrites in Drosophila by the BTB-zinc finger protein abrupt. Neuron. 2004;43:809–822. doi: 10.1016/j.neuron.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 10.Hummel T, Krukkert K, Roos J, Davis G, Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–370. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- 11.Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 12.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 13.Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development. 2003;130:5543–5552. doi: 10.1242/dev.00792. [DOI] [PubMed] [Google Scholar]
- 14.Yu W, Qiang L, Solowska JM, Karabay A, Korulu S, Baas PW. The Microtubule-severing Proteins Spastin and Katanin Participate Differently in the Formation of Axonal Branches. Mol Biol Cell. 2008;19:1485–1498. doi: 10.1091/mbc.E07-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]