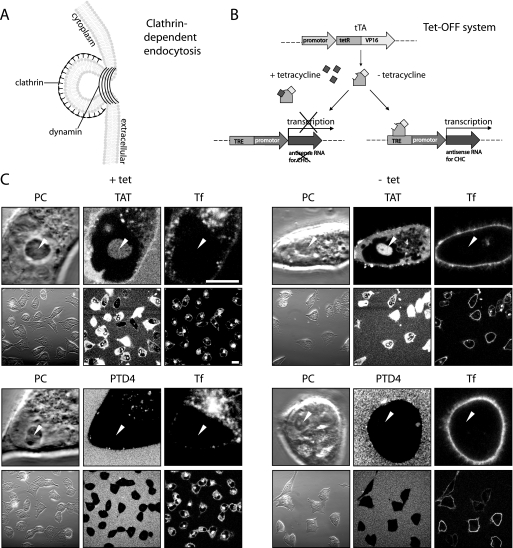
FIGURE 2.
Transduction of TAT is independent of clathrin-mediated endocytosis. A, schematic diagram illustrating clathrin-dependent endocytosis. The clathrin coat is required for membrane invagination, and for the scission of clathrin-coated vesicles dynamin is needed. B, schematic representation of the Tet-Off system, allowing a conditional knockdown of CHC in the BHK21-tTA/anti-CHC cell line. The binding of the transcriptional activator tTA to an operator sequence in the absence of tetracycline (–tet) results in activation of transcription of CHC-antisense RNA and thereby repression of the CHC mRNA translation. C, confocal optical sections of living cells during incubation with the fluorescent CPPs TAT (upper panel) or PTD4 (bottom panel) in the presence of the transferrin (Tf) as a marker for clathrin-dependent endocytosis. Each panel displays high magnification images of the phase contrast (PC) and the fluorescently labeled compound to show the details of their intracellular distribution and low magnification images to highlight the frequency of CPP transduction and Tf internalization (see also supplemental Fig. S3). Arrowheads mark the position of nucleoli. Transduction experiments were performed in the presence (left panel) and absence (right panel) of tetracycline. Although uptake of Tf is nearly abolished after tetracycline removal over a period of 6 days, the TAT CPP is still capable of reaching all intracellular compartments (diffuse, nonvesicular fluorescence, and accumulation inside nucleoli), indicating that this mode of uptake is not influenced by clathrin-dependent endocytosis. Vesicular uptake of the CPP TAT was still detected under –tet conditions albeit at reduced levels. Scale bar, 10 μm for high and 20 μm for low magnification images.