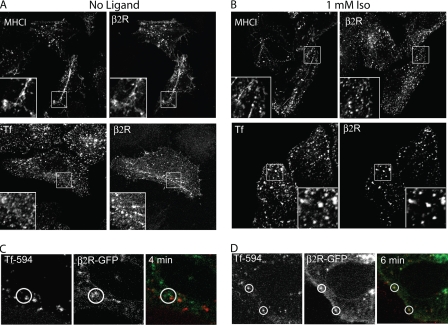
FIGURE 2.
β2 receptor internalization compared with MHCI and transferrin. A and B, HeLa cells were preincubated with anti-HA antibody (IgG1), and then incubated at 37 °C for 5 min without (A) or with ligand (B) in the presence of mouse anti-MHCI antibody (IgG2a) or Alexa 594-conjugated Tf. After fixation, unlabeled GAM antibody without saponin was used to block the remaining surface antibodies, and then isotype-specific antibodies 488-GAM-IgG1 and 594-GAM-IgG2a were used, in the presence of saponin to detect the internalized β2 receptor and MHCI, respectively. When Alexa 594-transferrin was present, we utilized only the secondary antibody 488-GAM (IgG1). Paired insets show magnified views and indicate the presence of the β2 receptor, without ligand, on tubular recycling endosomes. C, internalization of β2-GFP and Tf 594 in living HeLa cells in the absence of ligand. Still images (taken 4 min after Tf addition) from supplemental Movie S1. Without agonist, β2R-GFP and Tf 594 (red) were not observed together during the 15 min of incubation (see circled regions). D, internalization of β2-GFP compared with Tf 594 in living HeLa cells in the presence of (Iso, 1 mm). Still images (taken 6 min after addition of Tf and Iso) from supplemental Movie S2. Isoproterenol and Tf 594 were added at the same time. In the presence of isoproterenol, β2R-GFP, and Tf594 were present together in the same endosome (see circled regions). Images shown are representative of experiments that were repeated three times.