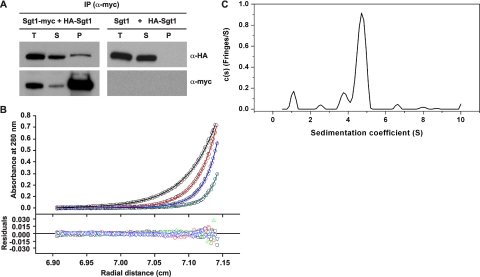
FIGURE 1.
Sgt1 forms dimers. A, Sgt1 associates with Sgt1 in vivo. Lysates of yeast cells that expressed HA-Sgt1 (plasmid pB1354) and either Sgt1-Myc (Y1649, left) or untagged Sgt1 (YPH499, right) were subjected to immunoprecipitation (IP) with anti-Myc antibodies. Immunoblot analyses were performed with anti-HA or anti-Myc antibodies. T, total lysates; S, supernatant; P, precipitate. B, an analytical ultracentrifugation of Sgt1 is shown. Absorbance scans at 280 nm at equilibrium are plotted versus the distance from the axis of rotation Sgt1. Shown is protein at a concentration of 5μm centrifuged at 4 °C for at least 24 h at 16,000 rpm (black), 20,000 rpm (red), 24,000 rpm (blue), and 30,000 rpm (cyan). The solid lines represent the best fit of all the data sets to a monomer-dimer self-association model with a dissociation equilibrium constant of 20 nm. The root mean square deviation of the fit is 0.0040 absorbance units. C, the sedimentation velocity profiles (fringe displacement) of Sgt1 were fitted to a continuous sedimentation coefficient distribution model (c(s)) as well as a hybrid continuous/discrete distribution model (not shown). The root mean square deviation of the fit is 0.018 fringes.