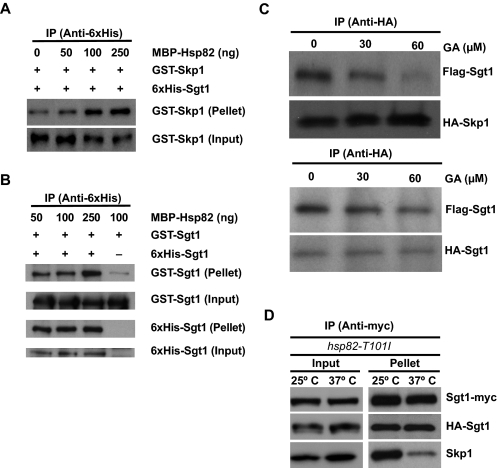
FIGURE 3.
Effect of Hsp90 on Sgt1 Dimerization. A, recombinant MBP-Hsp90 induces Sgt1-Skp1 binding. The indicated amounts of MBP-Hsp82 were added to ∼300 ng of His-Sgt1 and GST-Skp1 and incubated at 30 °C for 1 h before immunoprecipitation (IP) with anti-His antibodies. Input refers to total lysates (6% of starting material); Pellet refers to the immunoprecipitate. B, increased levels of Hsp82 stimulate Sgt1-Sgt1 binding moderately. The experiment is essentially the same as the one described in A, except that GST-Sgt1 was used in place of GST-Skp1. His-Sgt1 or GST-Sgt1 was detected by anti-His or anti-GST antibodies. C, Hsp90 is required for Sgt1-Skp1 binding but not Sgt1-Sgt1 binding in vitro. Upper, HA-Skp1-FLAG-Sgt1 binding was monitored in the presence of the Hsp90 inhibitor geldanamycin. In each reaction, equal amounts of HA-Skp1 and FLAG-Sgt1 proteins pretreated with DMSO or GA were immunoprecipitated with anti-HA antibody. Proteins in the immunoprecipitates were detected by tag-specific antibodies. Lower, similar to the experiment in the upper panel, except that HA-Sgt1 was used instead of HA-Skp1 in the Sgt1-Sgt1 binding assay. D, Sgt1-Sgt1 association is unaltered in hsp82 mutants, but Sgt1-Skp1 binding is reduced. Lysates were collected for the mutant strain hsp82-T101I (strain Y1653) that expressed HA-Sgt1 and Myc-Sgt1. Before lysis, the cells were grown either at 25 °C only or at 25 °C and shifted to 37 °C for 3 h. Lysates were subjected to immunoprecipitation with anti-Myc antibodies. Bound fractions were analyzed by using anti-HA, anti-Myc, and anti-Skp1 antibodies.