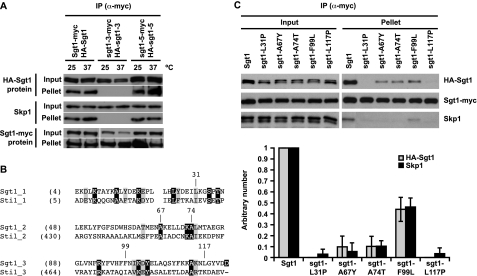
FIGURE 4.
The TPR domain of Sgt1 is important for its kinetochore function. A, the N-terminal region of Sgt1 that contains the TPR domain is important for Sgt1-Sgt1 and Sgt1-Skp1 interactions. HA-Sgt1, HA-sgt1-3, or HA-sgt1-5 was expressed in Sgt1-Myc, sgt1-3-Myc, or sgt1-5-Myc cells (Y1650, Y1651, and Y1652, respectively). Cells were either grown at 25 °C only or at 25 °C and shifted to 37 °C for 3 h before harvesting. The lysates were subjected to immunoprecipitation with anti-Myc antibodies conjugated to Sepharose. Anti-HA or anti-Myc antibodies were used to detect HA-tagged or Myc-tagged proteins, respectively, in the immunoprecipitates (IP). Skp1 was detected by anti-Skp1 antibodies. B, shown is an alignment of TPR motifs in Sgt1 with those in Sti1, a typical TPR protein (27). Identical residues are in black boxes; similar residues are in gray boxes; and the indicated residues, except for Ala-67, are those whose significance for the kinetochore function was described in our previous reports (21, 24). The starting amino acid position of each residue is shown in parentheses. C, all three TPR motifs of the Sgt1 TPR domain are important for kinetochore function. Strains (Y1662–1666 and Y1668) in which SGT1 was deleted were designed to express pRS414-HA-Sgt1 (plasmid pB180) and pRS415-Myc-Sgt1 (plasmid pB1367) or the indicated point mutations in pRS414-HA (plasmids pB1361–1365) and pRS415-Myc (plasmids pB1368–1372). Lysates were subjected to immunoprecipitation with anti-Myc antibodies conjugated to Sepharose (lower panels). Error bars indicate the S.D. of two independent results. The signals in the upper panels were quantified, and binding activity was defined as the ratio of the amount of coprecipitated protein to the amount of input protein. Specifically, the binding activity of wild-type Sgt1 was defined as 1, and the binding activity of each mutant was normalized to that value.