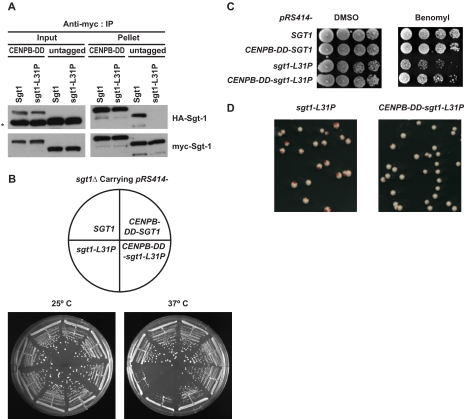
FIGURE 5.
Sgt1 dimerization is required for the kinetochore function. A, fusion of the CENP-B-DD with an Sgt1 mutant protein results in partial recovery of a lost phenotype. The 59 C-terminal amino acids of human CENP-B, in which the dimerization domain resides, were fused with Sgt1 (CENP-B-DD-Sgt1) and with Sgt1-L31P (CENP-B-DD-Sgt1-L31P). Both HA- and Myc-tagged constructs for each (wild-type and mutant) were expressed in sgt1Δ cells (Y1677 and Y1678, respectively). Untagged (without CENP-B-DD), HA-tagged, and Myc-tagged constructs were used for each (wild-type and mutant) and were expressed in sgt1Δ cells (Y1662 and Y1663, respectively). Cell lysate from all four strains were subjected to immunoprecipitation (IP) with anti-Myc antibodies conjugated to Sepharose. Anti-HA and anti-Myc antibodies were used to detect HA- and Myc-tagged CENP-B-DD-Sgt1, CENP-B-DD-Sgt1-L31P, Sgt1, and Sgt1-L31P. Skp1 was detected with anti-Skp1 antibodies. The asterisk indicates a nonspecific band. HA-Sgt1 and HA-Sgt1-L31P protein in Input lanes were very close to the nonspecific band. B, cells that expressed CENP-B-DD fused with Sgt1 (Y1675) or Sgt1-L31P (Y1676) were streaked on yeast extract-peptone-dextrose plates. The plates were also inoculated with a strain that expressed wild-type Sgt1 (Y1655) and a strain that expressed sgt1-L31P without CENP-B-DD (Y1656). Plates were incubated at 25 °C and 37 °C. Pictures were taken after 3 days of growth. C, the indicated strains (the same as in B) were grown on yeast extract-peptone-dextrose plates containing 15 mg/ml benomyl or DMSO only. The numbers of cells that were spotted onto each plate (left to right) were ∼5 × 104, 1 × 104, 2 × 103, and 4 × 102. The plates were incubated at 30 °C for 3 days. D, shown are the results of a colony color-sectoring assay to analyze the chromosome missegregation phenotype of cells expressing Sgt1-L31P (Y1670) and CENP-B-DD-Sgt1-L31P (Y1680).