Table 1.
Halogenation scope.a
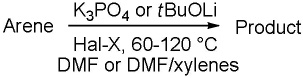 | ||||
|---|---|---|---|---|
| entry | arene | reagent base |
product | yield |
| 1 | 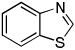 |
CCl4 tBuOLi |
80% | |
| 2 | 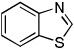 |
CBr4 K3PO4 |
82% | |
| 3 | CBr4 tBuOLi |
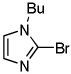 |
91% | |
| 4 | 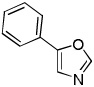 |
(BrCF2)2 tBuOLi |
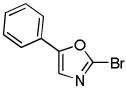 |
80% |
| 5 | 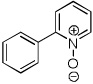 |
CBr4 tBuOLi |
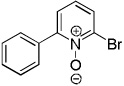 |
56% |
| 6b | CBr4 tBuOLi |
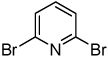 |
30% | |
| 7 | C6Cl5H | ICl tBuOLi |
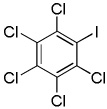 |
90% |
| 8 | 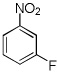 |
CBr4 tBuOLi |
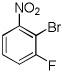 |
55% |
| 9 | 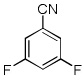 |
CBr4 tBuOLi |
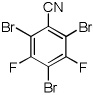 |
40% |
| 10 | C6F5H | I2 K3PO4 |
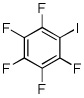 |
85% |
| 11 | 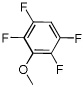 |
I2 tBuOLi |
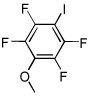 |
98% |
| 12 | 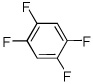 |
I2 tBuOLi |
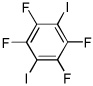 |
95% |
| 13c | 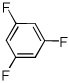 |
ICl tBuOLi |
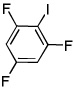 |
58% |
| 14d | 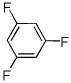 |
ICl tBuOLi |
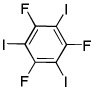 |
39% |
Substrate (1 equiv), base (2–4 equiv), halogenating reagent (1.5-4 equiv). Yields are isolated yields. See the Supporting Information for details.
m-Xylene solvent.
1,3,5-Trifluorobenzene (3 equiv), ICl (1 equiv).
1,3,5-Trifluorobenzene (1 equiv), ICl (4 equiv).
