Table 1.
Scope of alkynyl ether sythesis via elimination of enol triflatesa
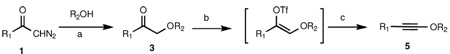 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | 3 (% yield) | 5 | 5 (% yield) |
| 1 | Ph | menthyl | 91 | a | 75 |
| 2 | Ph | CH2CH(CH2)3 | 89 | b | 82 |
| 3 | Ph | t-Bu | 67 | c | 68 |
| 4 | Ph | Ph | 78 | d | 70 |
| 5 | 1-cyclohexenyl | menthyl | 84 | e | 90 |
| 6 | (CH3)2CCH | CH2CH(CH2)3 | 81 | f | 68 |
| 7 | PhCC | menthyl | 69b | g | 64 |
| 8 | t-Bu | CH2CH(CH2)3 | 87 | h | --c |
| 9 | C6H13 | menthyl | 90 | i | --c |
Reaction conditions: a. R2OH (1.5 equiv), In(OTf)3 (10 mol %), toluene, rt; b. LiHMDS, THF, −78 °C, then PhNTf2, DMPU, −78 °C-rt; c. KOt-Bu, THF, −78 °C.
Yield of 3g prepared in three steps from menthol via an alternative route described in the text.
No alkynyl ethers were detected in the reaction mixture; see text for details.
