Table 2.
Scope of alkynyl ether synthesis via elimination of enol phosphatesa
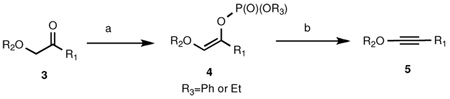 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R2 | 4 | % yield 4 | 5 | % yield 5 |
| 1 | t-Bu | CH2CH(CH2)3 | hb | 81 | h | 60 |
| 2 | C6H13 | menthyl | ic | 89 | i | 72 |
| 3 | i-Pr | menthyl | jb | 78 | j | 85 |
| 4 | 1-cyclohexenyl | t-Bu | kb | 72 | k | 70 |
| 5 | Ph | t-Bu | lb | 82 | c | 56 |
Reaction conditions: a. KHMDS, ClPO(OR3)2, THF, 78 °C; b. n-BuLi, KOt-Bu, THF, −78 °C.
The diethylphosphate derivative was synthesized.
The diphenylphosphate derivative was synthesized.
