Figure 1.
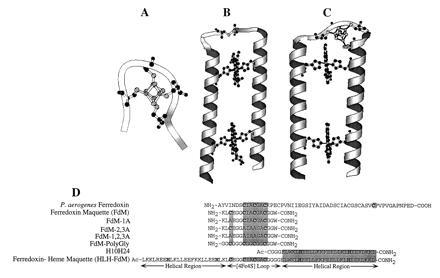
Modular design of the ferredoxin maquette. (A) molscript (13) model of the monomeric hexadecapeptide with bound [4Fe–4S]. (B) Molecular model of the prototype heme–protein maquette H10H24. (C) Computer representation of the modular design helix–loop–helix ferredoxin–heme maquette (HLH–FdM) with a [4Fe–4S] cluster and a pair of bound hemes. (D) Primary sequence alignment illustrating the modular peptide design approach. Given are the sequences for Peptococcus aerogenes Fd (from which the [4Fe–4S]-binding domain was extracted), the natural sequence ferredoxin maquette FdM, versions of the ferredoxin maquette with one, two, and three cysteines to alanine modifications, the glycine-modified ferredoxin maquette, and the HLH–FdM. All synthetic peptides were C-terminally amidated, and the HLH–FdM was N-terminally acetylated.
