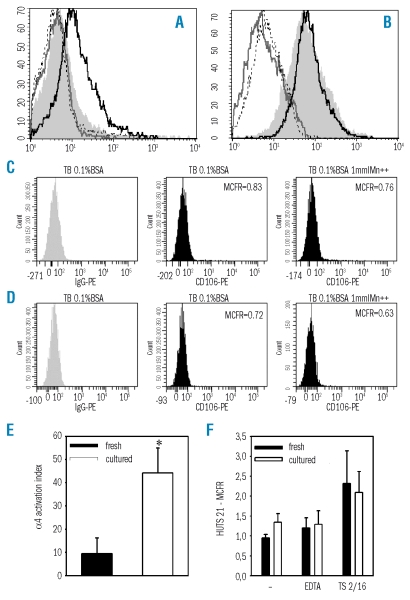
Figure 3.
Activation state of α4 and β1 integrins on fresh or expanded CD34+ cells. (A) Fresh or (B) expanded CD34+ cells were resuspended in Tyrode’s buffer 1% BSA (shaded histogram) or Tyrode’s buffer 1% BSA supplemented with 5 mmol/L EDTA (gray line), with 1 mmol/L MnCl2 (black line), or with 1 mmol/L MnCl2 together with function-blocking anti-α4 monoclonal antibody P4C2 (dotted line). Soluble VCAM-1 was added and cells were incubated for 3 hours at 37°C. Binding was measured by cell staining with phycoerythrin (PE)-conjugated anti-VCAM-1 (anti-CD106) monoclonal antibody. Results of one representative experiment out of four are shown. As compared to IgG-PE, binding of CD106 was undetectable in fresh (C) or cultured CD34+ cells (D) which had not been previously exposed to soluble VCAM-1, either in Tyrode’s buffer (TB) 0.1% BSA or in TB supplemented with 1 mmol/L MnCL2. (E) The activation index of α4 integrin on fresh and expanded CD34+ cells was calculated as 100 × [(Fo−Fr)/(Fmax−Fr)], where Fo is the mean fluorescence intensity of soluble VCAM-1 binding in Tyrode’s buffer 1% BSA, Fr is background fluorescence in the presence of 5 mmol/L EDTA, and Fmax is the fluorescence intensity in the presence of 1 mmol/L MnCl2. *p<0.05, n=4. (F) Fresh or expanded CD34+ cells were stained with HUTS-21 monoclonal antibody which is directed against an activation epitope of β1 integrin. HUTS-21 expression was measured in cells incubated in Tyrode’s buffer (−), in Tyrode’s buffer supplemented with 5 mmol/L EDTA or with β1 activating monoclonal antibody TS2/16 (n=3).