Abstract
Various guidelines have been proposed regarding which portions of a surgical gown may be considered sterile. Unfortunately, the validity of these recommendations has not been definitively established. We therefore evaluated gown sterility after major spinal surgery to assess the legitimacy of these guidelines. We used sterile culture swabs to obtain samples of gown fronts at 6-inch increments and at the elbow creases of 50 gowns at the end of 29 spinal operations. Another 50 gowns were swabbed immediately after they were applied to serve as negative controls. Bacterial growth was assessed using semiquantitative plating techniques on a nonselective, broad-spectrum media. Contamination was observed at all locations of the gown with rates ranging from 6% to 48%. Compared with the negative controls, the contamination rates were greater at levels 24 inches or less and 48 inches or more relative to the ground and at the elbow creases. The section between the chest and operative field had the lowest contamination rates. Based on these results, we consider the region between the chest and operative field to be the most sterile and any contact with the gown outside this area, including the elbow creases, should be avoided to reduce the risk of infection.
Introduction
Every surgical intervention is associated with an inherent risk of postoperative wound infection. Although relatively uncommon, this complication may dramatically alter the clinical course of an affected patient. As a result, every effort is made by the surgeon and operating room staff to adhere to proper sterile techniques to prevent surgical-site infections.
Contamination of the operative field is a major concern to all orthopaedic surgeons. However, spinal procedures may be more susceptible to bacterial seeding because they frequently involve extensile approaches with considerable disruption of the soft tissue envelope, prolonged surgical times, and implantation of instrumentation, all of which increase infection rates. For example, the incidence of infection after lumbar discectomy is less than 1% [17, 27] compared with 1% to 5% for noninstrumented lumbar fusions [17, 27] and 7% for instrumented constructs [17]. In addition to the greater scale and length of the surgical intervention, other factors associated with a higher risk of surgical-site contamination include the use of power equipment such as a high-speed burr [24], and larger number of personnel present in the operating room [17, 20, 21]. Ritter et al. reported a 35-fold increase in colony-forming units per hour in an empty operating room versus when five people were present [20, 21].
Surgical instruments and other equipment used in the operative field with reported contamination rates are light handles (0% to 14%) [5, 12], sucker tips (11% to 41%) [5, 10, 22, 23], scalpel blades (9%) [5], and fascial needles (10%) [5]. Similar studies assessing the sterility of operating room staff have been performed: glove contamination has been documented in 14% to 57% of orthopaedic cases depending on the subspeciality [2, 5, 6, 15], and in previous reviews, the overall incidence of glove perforation ranged between 9% and 37% [6, 26, 30].
To minimize the risk of surgical-site contamination, guidelines have been presented regarding which portions of a gown may be considered sterile. The Association of Perioperative Registered Nurses (AORN) suggests the front of the gown is sterile between the levels of the chest and the operative field [2]; nevertheless, this is an empiric recommendation that is not based on specific data (e-mail communication with AORN—Ramona Conner, 24 January 2008). Others stipulate the area immediately below the neckline or the axillae may represent the upper limits of the sterile zone [3, 25, 28]. Similarly, each of these resources further recommends the sterile zone on the gown sleeve should extend above the elbow crease. For obvious reasons, it is critical that appropriate evidence-based preventive measures be adopted to address any potential breaks in sterile technique that may occur during surgical intervention. To reliably accomplish this goal, the boundaries of the sterile field must be clearly defined; however, at this time, the relative risks of intraoperative contamination associated with specific regions of the surgical gown have not been characterized.
We therefore asked which sections of the surgical gown should be considered most sterile by measuring the rate of postoperative contamination at various gown heights and specific associated body regions after spinal procedures. These postoperative contamination rates then were compared with preoperative control group contamination rates, and the most sterile zone was defined as that where we identified no differences between preoperative and postoperative contamination rates.
Materials and Methods
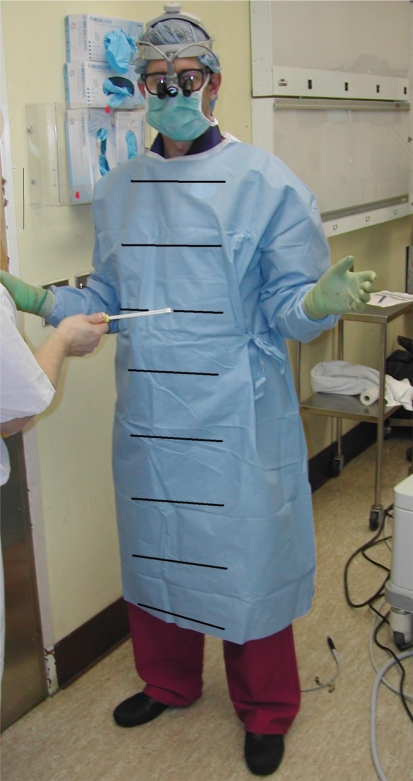
We acquired cultures from 50 sterile disposable surgical gowns (Impervious Surgical Gown, AAMI Level 4; Cardinal Health, McGaw Park, IL) from a series of 29 surgical cases performed by two spine surgeons (PWG, JNG). We routinely use disposable gowns in our institution because they are more impermeable to blood and other fluids compared with reusable gowns [9, 19]. Using sterile swabs, we cultured the front of each experimental gown at 6-inch increments starting from a location 18 inches above the ground and continuing cephalad to include the neckline (Fig. 1). We collected all samples at the completion of each operation before the surgical team had knowingly contaminated themselves and removed their gowns. At each sampling point, the entire width of the gown front was sampled taking care not to contact the more lateral portions. We also sampled both elbow creases starting from two inches below and up to two inches above the elbow crease. For each set of cultures, a swab of the underlying scrubs or lead apron was used as a positive control. We excluded cultures of any staff that participated in application of dressing or removal of the surgical drapes from the patient, as such activities expose individuals to unsterile regions on the operative field or patient’s skin.
Fig. 1.
The regions of gowns, excluding the elbow creases, where culture samples were collected (lines digitally added) are shown.
We then assessed an additional 50 gowns as negative controls, using the techniques described previously to confirm their sterility before being exposed to any sources of intraoperative contamination. For each of these negative controls, culture swabs were obtained immediately after the gown was applied in the usual way and before an individual would have entered the surgical field. We recorded the following data for each individual: total height and the distances from the floor to the shoulders, axillae, chest (nipple line), waist, and knees; level of the operating room table; duration the subject was in the sterile field; whether a lead apron was worn under the gown; type of surgery; and total number of personnel scrubbed during the procedure.
Culture swabs therefore were available for 50 experimental and 50 control gowns. The surgical cases from which the swabs were obtained, the mean values for the duration of time that the surgical gowns were used, height of the operating room table, total height of each individual, and number of people scrubbed during the operation were recorded (Tables 1, 2). In addition, the percentage of subjects who wore a lead apron under their gowns and the identifying data of the personnel who were sampled also were recorded (Table 2).
Table 1.
Cases from which culture swabs were obtained
| Region and surgical approach | Percentage of gowns |
|---|---|
| Cervical spine, anterior approach | 18% |
| Cervical spine, posterior approach | 20% |
| Thoracolumbar, anterior approach | 8% |
| Thoracolumbar, posterior approach | 54% |
Table 2.
Personnel and operating room characteristics
| Surgical environment | Average (SD) |
|---|---|
| Duration gowns worn (minutes) | 134 (67) |
| Individuals’ total height (inches) | 68.0 (4.1) |
| Table height (inches) | 33.4 (1.5) |
| Total number of personnel scrubbed per case | 3.54 (0.54) |
| Specific personnel | Percentage of gowns |
|---|---|
| Surgical attending | 44% |
| Orthopaedic chief resident/spine fellow | 30% |
| Surgical technician | 26% |
| Gowns with lead apron underneath | 82% |
SD = standard deviation.
Cultures were procured using the following protocol; the tips of sterile culture swabs (StarSwab II™ with liquid Stuart’s medium; Starplex Scientific Inc, Etobicoke, Ontario, Canada) initially were dipped in sterile saline to facilitate extraction of any contaminants present on the gown surface. The swabs were shaken gently to remove any excess fluid before being used to sample the gowns.
As part of a well-accepted semiquantitative technique, these swabs were streaked on one quadrant of a 5% sheep blood Columbia agar plate (Remel Inc, Lenexa, KS), which is a standard, nonselective media that is known to support the growth of numerous bacterial strains, including Gram-negative and Gram-positive species (eg, Staphylococci and Streptococci) [7]. We achieved successive dilutions by streaking the remaining three quadrants in succession, making sure to use a new sterile, disposable loop for each streaking maneuver.
Plates were incubated at 37°C for 48 hours, after which they were evaluated for the presence of bacterial colonies; we graded each sample on a scale of 0 to 4+ based on the number of quadrants on each plate that showed positive growth. As reported previously, any growth pattern of 1+ or higher was considered consistent with contamination [4, 11, 14].
To determine if a significant rate of contamination occurred at each 6-inch location on the gown, we performed a separate Fisher’s exact test to compare the contamination rate of postoperative swabs with that observed for negative control swabs. Second, all cultures were pooled according to which of six regions of the gown the sample was collected from: above the axilla, axilla to the chest, chest to the waist, waist to the operating room table, operating room table to the knees, or below the knees. We then compared the collective postoperative contamination rate of each of these groups with the rate of corresponding negative control swabs using a Fisher’s exact test. Similarly, the contamination rates of the elbow crease swabs were compared with those of negative controls. We used SPSS 16.0 software (SPSS, Chicago, IL) for all analyses.
Results
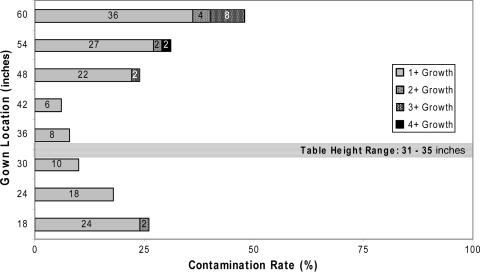
Contamination or growth was observed in one of the 50 (2%) negative control groups: one plate at the 18-inch location near the bottom edge of the gown showed 1+ growth. Conversely, all 50 (100%) of the positive controls derived from preoperative and postoperative samplings showed growth. In the postoperative group, contamination was detected at all 6-inch locations on the gown with contamination rates ranging from 6% to 48% (Table 3). Postoperative contamination rates at l24 inches or less (ie, 18, 24 inches) and 48 inches or greater (ie, 48, 54, 60 inches) relative to the ground were greater than those of the negative controls (Fig. 2), whereas such a difference was not evident at the 30-, 36-, and 42-inch locations.
Table 3.
Contamination rates of gowns
| Contamination rates (%) by height on gown (inches) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial growth | Preoperative contamination rates | ||||||||
| 18 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | (+) Control | |
| 1+ | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 38 |
| 3+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 62 |
| 4+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Bacterial growth | Postoperative contamination rates | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 18 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | (+) Control | |
| 1+ | 24 | 18 | 10 | 8 | 6 | 22 | 27 | 36 | 5 |
| 2+ | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 48 |
| 3+ | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 8 | 38 |
| 4+ | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 9 |
| Total | 26 | 18 | 10 | 8 | 6 | 24 | 31 | 48 | 100 |
| p value | 0.001 | 0.003 | 0.056 | 0.117 | 0.242 | 0.0002 | < 0.0001 | < 0.0001 | 1.00 |
Fig. 2.
Contamination rates ranged from 6% to 48% for surgical gowns after use.
Bacterial growth was observed most frequently in the areas above the chest (33%–42%) and below the operating room table (17%–22%); contamination rates of these sections were greater than those of the negative controls (Table 4). In contrast, the portion of the gown between the chest and the operating room table had the lowest contamination rates (6%–9%). Likewise, 18% of swabs acquired from the elbow creases were contaminated after surgery, a percentage that also was greater (p = 0.003) than the value associated with the corresponding negative controls.
Table 4.
Contamination rates for regions of surgical gowns
| Contamination rate | Region of gown | |||||
|---|---|---|---|---|---|---|
| Below knee | Table to knee | Waist to table | Chest to waist | Axilla to chest | Above axilla | |
| Preoperative | 1% | 0% | 0% | 0% | 0% | 0% |
| Postoperative | 22% | 17% | 9% | 6% | 33% | 42% |
| p value | 0.0001 | 0.00005 | 0.057 | 0.060 | 0.0002 | < 0.00001 |
Discussion
Minimizing the incidence of surgical-site contamination during orthopaedic procedures is a major priority because development of a postoperative infection is a potentially devastating complication. Given the relatively prolonged surgical times and frequent inclusion of metal implants, spinal operations may be particularly predisposed to bacterial seeding of the wound. The Centers for Disease Control (CDC) currently recommends wearing a surgical mask covering the nose and mouth, along with a cap or hood covering scalp and facial hair [16]. Furthermore, as no study has directly examined gown contamination and the risk of surgical-site infection, the only recommendation that the CDC currently has, with respect to gowns, is that they should be impermeable to liquids and viruses [16]. Among the numerous precautions that have been implemented to reduce contamination rates, the majority of surgeons would agree that selected regions of the gown are not sterile and should not be in direct contact with the operative field; nevertheless, the validity of these empiric guidelines have not been definitively established. We attempted to better define which sections of the surgical gown should be considered most sterile by measuring the rate of postoperative contamination at various gown heights and specific associated body regions after spinal procedures.
We acknowledge certain limitations to this study. We did not obtain negative control swabs immediately before each operation because we did not believe it appropriate to delay the procedures so members of the surgical team could be sampled. Further, baseline cultures might increase the potential risk of inadvertent gown contamination during this process. We evaluated cultures only for positive growth, and specific bacterial isolates were not identified, and therefore we cannot comment on the clinical relevance of the isolates: some might be unimportant. We did use a broad-spectrum media to assess bacterial growth and to better capture contaminates. Finally, the sample size of each cohort consisted of only 50 surgical gowns, so it is conceivable with greater power we would have observed differences between contamination rates of the postoperative and negative control swabs collected at the level of the surgical field. We do not believe these points appreciably detract from the overall importance of our results and conclusions.
Despite adherence to standard aseptic surgical techniques, our findings indicate that although bacterial contamination may be identified in all areas of the surgical gown at the completion of surgical cases, certain portions of the gown clearly were involved more frequently than others. According to these experiments, cultures obtained from the gown below the surgical table (18 and 24 inches relative to the ground) and above chest level (48, 54, and 60 inches) showed considerably greater bacterial growth than the respective negative controls. We suspect sterility of the chest region may have been compromised by bacterial shedding from the individual’s head or mask, both of which are in close proximity to the upper part of the surgical gown. It also may be assumed that the portion of the gown below the operating room table most likely becomes contaminated from direct contact with any number of unsterile objects situated below the draped field. Moreover, the axillae may be exposed to greater amounts of perspiration, which would be expected to attenuate the impervious properties of the gown in this location [18]. These specific areas are considered unsterile based on recommendations provided by the AORN [2].
The sterility of the gown sleeves extends proximally to 2 inches above the elbow crease [2, 3, 25, 28]. We found elbow creases had a high contamination rate of 18% compared with that of negative control swabs. As with the axillae, the elbows may be subject to the deleterious effects of perspiration, which again may attenuate the impervious properties of the gown in this location [18]. Also, additional breaks in the sterility of this region may occur as the arms are tucked to the side so the elbows are positioned close to the side and rear portions of the gown.
We observed no differences in contamination rates for the section of the gown between the chest and table suggesting this segment is at decreased risk for contamination. However, bacterial growth was documented in this region, confirming that for even the most sterile parts of the gown, the risk of transmitting infectious agents to the surgical field is not negligible.
Although various studies have attempted to characterize which portions of the gown may be considered sterile, none substantiate the claims. Based on our data, we regard the front of the gown between the operative table and the chest to be the area of greatest sterility and we strongly advise scrubbed personnel to avoid contacting the surgical field with their elbow creases to minimize the risk of infection.
Acknowledgments
We thank Debbie Callan and Patty Farrel for invaluable assistance with microbiology techniques.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Al-Maiyah M, Bajwa A, Mackenney P, Port A, Gregg PJ, Hill D, Finn P. Glove perforation and contamination in primary total hip arthroplasty. J Bone Joint Surg Br. 2005;87:556–559. [DOI] [PubMed]
- 2.AORN Recommended Practices Committee. Recommended practices for maintaining a sterile field. AORN J. 2006;83:402–404, 407–410, 413–416. [DOI] [PubMed]
- 3.Boess-Lott R, Stecik S. The Ophthalmic Surgical Assistant. 1st Ed. Thorofare, NJ: Slack Inc; 1999.
- 4.Boots RJ, Howe S, George N, Harris FM, Faoagali J. Clinical utility of hygroscopic heat and moisture exchangers in intensive care patients. Crit Care Med. 1997;25:1707–1712. [DOI] [PubMed]
- 5.Davis N, Curry A, Gambhir AK, Panigrahi H, Walker CR, Wilkins EG, Worsley MA, Kay PR. Intraoperative bacterial contamination in operations for joint replacement. J Bone Joint Surg Br. 1999;81:886–889. [DOI] [PubMed]
- 6.Eckersley JR, Williamson DM. Glove punctures in an orthopaedic trauma unit. Injury. 1990;21:177–178. [DOI] [PubMed]
- 7.Ellner PD, Stoessel CJ, Drakeford E, Vasi F. A new culture medium for medical bacteriology. Am J Clin Pathol. 1966;45:502–504. [DOI] [PubMed]
- 8.Fang A, Hu SS, Endres N, Bradford DS. Risk factors for infection after spinal surgery. Spine. 2005;30:1460–1465. [DOI] [PubMed]
- 9.Granzow JW, Smith JW, Nichols RL, Waterman RS, Muzik AC. Evaluation of the protective value of hospital gowns against blood strike-through and methicillin-resistant Staphylococcus aureus penetration. Am J Infect Control. 1998;26:85–93. [DOI] [PubMed]
- 10.Greenough CG. An investigation into contamination of operative suction. J Bone Joint Surg Br. 1986;68:151–153. [DOI] [PubMed]
- 11.Herruzo-Cabrera R, Vizcaino-Alcaide MJ, Pinedo-Castillo C, Rey-Calero J. Diagnosis of local infection of a burn by semiquantitative culture of the eschar surface. J Burn Care Rehabil. 1992;13:639–641. [DOI] [PubMed]
- 12.Hussein JR, Villar RN, Gray AJ, Farrington M. Use of light handles in the laminar flow operating theatre: is it a cause of bacterial concern? Ann R Coll Surg Engl. 2001;83:353–354. [PMC free article] [PubMed]
- 13.Keller RB, Pappas AM. Infection after spinal fusion using internal fixation instrumentation. Orthop Clin North Am. 1972;3:99–111. [PubMed]
- 14.Linares J, Dominguez MA, Martin R. Current laboratory techniques in the diagnosis of catheter-related infections. Nutrition. 1997;13(4 suppl):10S–14S. [DOI] [PubMed]
- 15.Maffulli N, Capasso G, Testa V. Glove perforation in pediatric orthopaedic surgery. J Pediatr Orthop. 1991;11:25–27. [DOI] [PubMed]
- 16.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97–132; quiz 133–134; discussion 96. [DOI] [PubMed]
- 17.Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99–108. [PubMed]
- 18.Mills SJ, Holland DJ, Hardy AE. Operative field contamination by the sweating surgeon. Aust N Z J Surg. 2000;70:837–839. [DOI] [PubMed]
- 19.Pissiotis CA, Komborozos V, Papoutsi C, Skrekas G. Factors that influence the effectiveness of surgical gowns in the operating theatre. Eur J Surg. 1997;163:597–604. [PubMed]
- 20.Ritter MA. Surgical wound environment. Clin Orthop Relat Res. 1984;190:11–13. [PubMed]
- 21.Ritter MA, French ML, Hart JB. Microbiological studies in a horizontal wall-less laminar air-flow operating room during actual surgery. Clin Orthop Relat Res. 1973;97:16–18. [DOI] [PubMed]
- 22.Robinson AH, Drew S, Anderson J, Bentley G, Ridgway GL. Suction tip contamination in the ultraclean-air operating theatre. Ann R Coll Surg Engl. 1993;75:254–256. [PMC free article] [PubMed]
- 23.Sankar B, Ray P, Rai J. Suction drain tip culture in orthopaedic surgery: a prospective study of 214 clean operations. Int Orthop. 2004;28:311–314. [DOI] [PMC free article] [PubMed]
- 24.Schultz RB, Probe RA, Holmes GP. Contamination risks from a high-speed bone burr. Spine. 1996;21:1796–1797. [DOI] [PubMed]
- 25.Spry C. Essentials of Perioperative Nursing. 2nd Ed. Gaithersburg, MD: Aspen Publishers; 1997.
- 26.Thanni LO, Yinusa W. Incidence of glove failure during orthopedic operations and the protective effect of double gloves. J Natl Med Assoc. 2003;95:1184–1188. [PMC free article] [PubMed]
- 27.Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord. 2000;13:422–426. [DOI] [PubMed]
- 28.Whalan C. Assisting at Surgical Operations: A Practical Guide. Cambridge, UK: Cambridge University Press; 2006.
- 29.Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord. 1998;11:124–128. [PubMed]
- 30.Yinusa W, Li YH, Chow W, Ho WY, Leong JC. Glove punctures in orthopaedic surgery. Int Orthop. 2004;28:36–39. [DOI] [PMC free article] [PubMed]