Figure 8. Model for the fate of kinase clients and the effects of HSP90 inhibition or targeting CDC37.
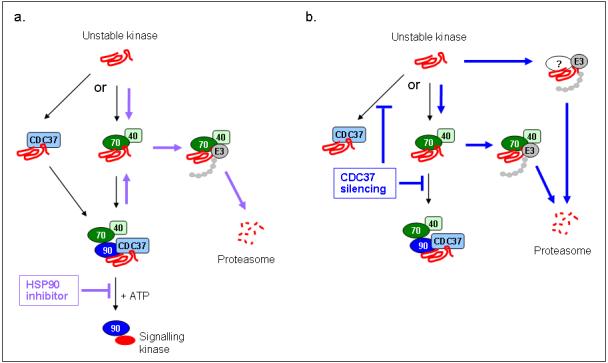
(a) Based on the results in this paper, there may be (at least) two possible routes of recruitment to HSP90: kinases either associate with HSC70/HSP40 and then form a stable complex with HSP90/CDC37, or may associate directly with CDC37 and are then recruited to HSP90. Under normal conditions the kinase is stabilised and/or rendered competent for activation by the ATP-driven HSP90 chaperone cycle, permitting kinase signalling. Treatment with an ATP-competitive HSP90 inhibitor blocks the conformational modification of kinases bound to HSP90, which in an unfolded state may return to an HSC70/HSP40 complex. The association of ubiquitinating enzymes (eg. E3) with HSC70 facilitates polyubiquitination of the unfolded kinase, which is subsequently degraded via the proteasome. (b) By silencing CDC37 expression, the binding of the unstable kinase to HSP90 is reduced. This may leave the unstable kinase prone to factors that directly target it for degradation or it may be ubiquitinated in the HSC70/HSP40 complex which is coupled to the proteasome degradation machinery. Black arrows indicate routes under normal conditions, purple arrows indicate proposed routes during HSP90 inhibition and blue arrows indicate proposed routes during CDC37 silencing. Note that the pulse chase experiment (Fig 7c) indicates that, at least in the case of CDK4, CDC37 depletion appears to result in degradation of the mature but unstable kinase, whereas there is evidence that HSP90 inhibition leads to degradation of newly synthesised client (Stepanova et al. 1996).
