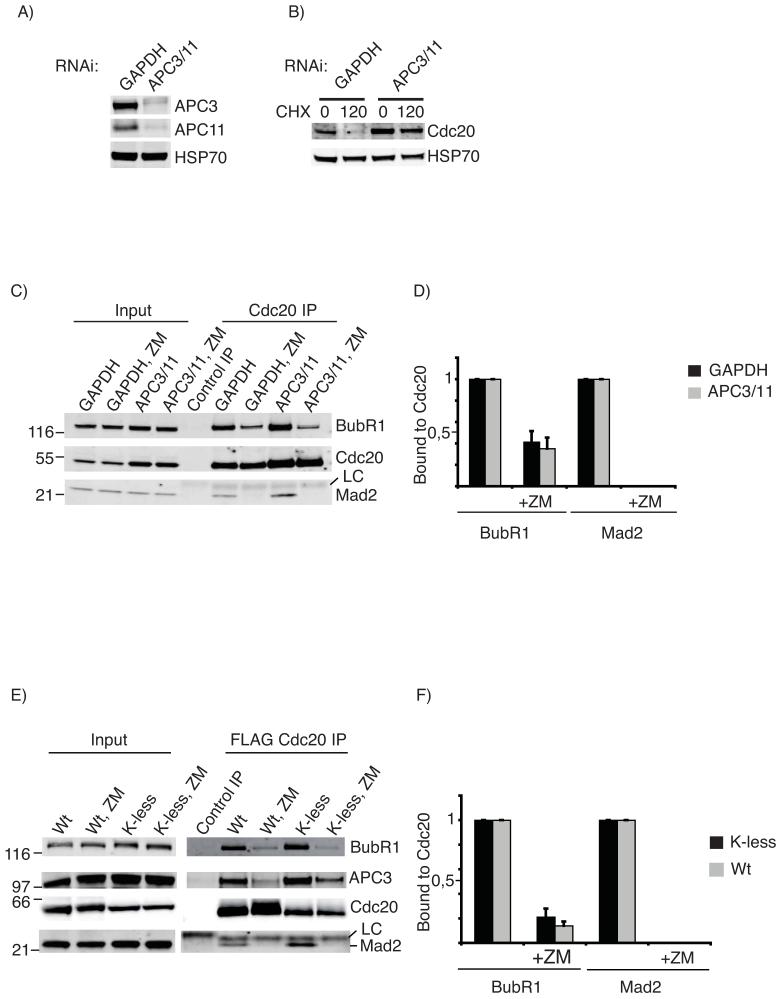
Figure 6.
Dissociation of checkpoint proteins from Cdc20 does not require ubiquitination of Cdc20.
A) Cells were treated with siRNA against GAPDH or siRNAs against APC3 and APC11 and immunoblotted for APC3, APC11, and Hsp70 as a loading control. B) Cells were treated as in A), arrested in mitosis with taxol and the half-life of Cdc20 assayed by adding cycloheximide (CHX). C) Cells treated as in A) were incubated with Taxol for 6 hrs, the samples divided in two and MG132 or MG132 plus ZM447439 added. After 2 hrs Cdc20 was immunopurified, and the amount of BubR1 and Mad2 bound to Cdc20 determined by quantitative immunobloting. (LC= antibody light chain). The whole blot is shown in Fig S7. Note the blot was cut into 3 and panels probed for BubR1 or Cdc20 or Mad2. D) Quantification of the experiment shown in C: the level of bound BubR1 and Mad2 is normalized to the amount of Cdc20, and the level of BubR1 and Mad2 set to 1 in the samples without ZM447439. The mean +/- SD of 3 independent experiments is shown.
E) HeLa cell lines stably expressing FLAG-tagged wt or K-less Cdc20 were released from S phase, MG132 added 9 hrs later for 2 hrs and mitotic cells harvested by shake-off. Samples were split in two and 100 nM Taxol plus MG132 added to both. After 1 hr FLAG-Cdc20 was immunopurified from one and 4 μM ZM447439 added to the other 1.5 hrs before immunoprecipitating FLAG-Cdc20. The amount of BubR1, APC3 and Mad2 bound to Cdc20 was determined by quantitative immunobloting. F) Quantification of the experiment shown in E) with the level of BubR1 and Mad2 normalized to the amount of Cdc20 and the amount of BubR1 and Mad2 bound to Cdc20 set to 1 in experiments without ZM447439. Note that the K-less mutant of Cdc20 co-immunopurifies from taxol-treated cells with more BubR1 and Mad2 than does wt Cdc20. The mean +/- SD of 3 independent experiments is shown. The amount of APC3 associated with K-less and wt Cdc20 dropped by 75% when the checkpoint was inactivated.