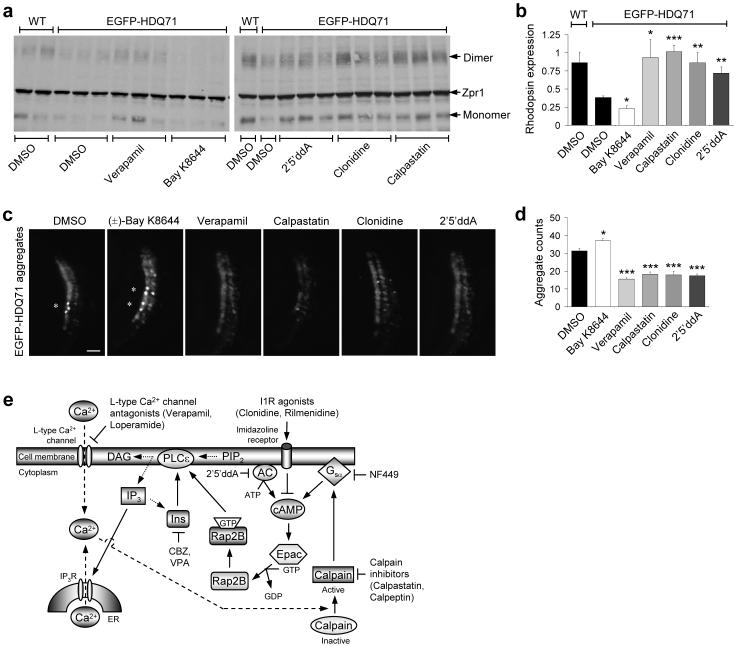
Figure 8. L-type Ca2+ channel antagonists, imidazoline-1 receptor agonists, cAMP antagonists and calpain inhibitors rescue Huntington’s disease phenotypes in zebrafish.
a, Western blot showing EGFP-HDQ71-induced degeneration of rod photoreceptors at 9 d.p.f. as indicated by the lack of monomeric (∼36 kDa) and dimeric rhodopsin (∼72 kDa), that is further reduced by 3 μM (±)-Bay K8644, and recovered upon treatment with 3 μM verapamil, 100 μM 2′5′ ddA, 3 μM clonidine and 1 μM calpastatin. No general toxicity to the zebrafish at these concentrations was observed.
b, Expression of EGFP-HDQ71 significantly reduces the levels of rhodopsin expression, when compared to wild-type (WT), non-transgenic controls. This affect is further abrogated by 3 μM (±)-BayK8644, while 3 μM verapamil, 100 μM 2′5′ddA, 3 μM clonidine and 1 μM calpastatin all protected against rod photoreceptor degeneration. Error bars: Standard error of mean.
c, Magnified bright field and EGFP images of zebrafish eye sections, taken at the ventral marginal zone for each of the indicated treatments (with concentrations as in Fig. 8b), are shown. Bar, 10 μm.
d, The total number of aggregates across 8×12 μm sections were counted and compared to the EGFP-HDQ71 DMSO-treated control. 3 μM (±)-Bay K8644 was found to significantly increase the aggregate burden, while 3 μM verapamil, 1 μM calpastatin, 3 μM clonidine and 100 μM 2′5′ddA decreased the aggregate load. As in our cell models, these aggregates were insoluble in 4% triton/SDS. Error bars: Standard error of mean. ***, p<0.001; **, p<0.01; *, p<0.05.
e, Schematic representation of an mTOR-independent autophagy pathway involving cAMP-Ca2+-calpains-Gsα with multiple drug targets. Intracellular cAMP levels are increased by adenylyl cyclase (AC) activity, thereby activating Epac which subsequently activates Rap2B, a small G-protein that activates PLC-ε resulting in the production of IP3 and consequent Ca2+ release from the ER. Intracellular Ca2+ levels are also increased by L-type Ca2+ channel agonists. An increase in intracytosolic Ca2+ activates calpains, which cleave and activate Gsα that activates adenylyl cyclase to increase cAMP levels, thereby forming a loop. Activation of this pathway inhibits autophagy. Multiple drug targets acting at various places in this pathway induce autophagy, such as imidazoline-1 receptors (I1R) agonists (clonidine and rilmenidine) and adenylyl cyclase inhibitor (2′5′ddA) that decrease cAMP levels, agents lowering inositol and IP3 levels [carbamazepine (CBZ) and valproic acid (VPA)]11, L-type Ca2+ channel antagonists (verapamil and loperamide), calpain inhibitors (calpastatin and calpeptin) and Gsα inhibitor (NF449).