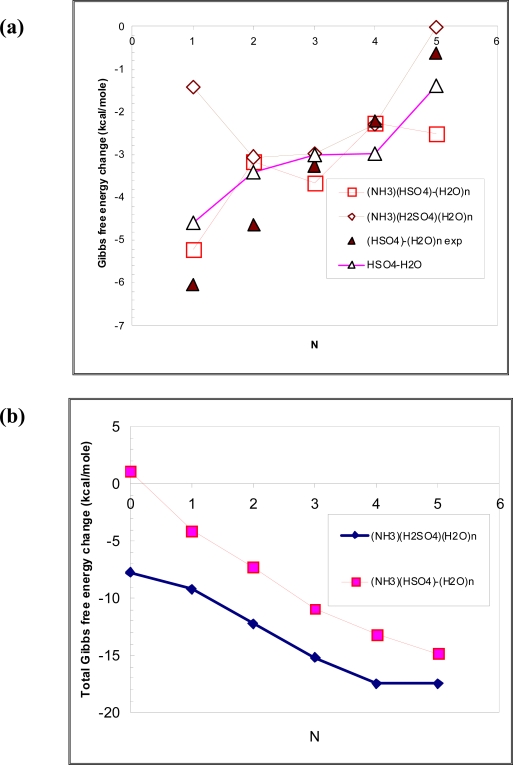
Figure 2.
The comparison of: (a) the stepwise Gibbs free energy changes associated with the hydration of (HSO4−)(H2O)n. [35], (H2SO4)(NH3)(H2O)n [21] and (HSO4−)(NH3)(H2O)n (present study) and (b) total free energies associated with the formation of (H2SO4)(NH3)(H2O)n [21] and (HSO4−)(NH3)(H2O)n from (H2SO4), (NH3) and water molecules and (H2O)n and (HSO4−), (NH3) and water molecules, respectively. T = 298.15 K and P = 101.3 KPa. Subscript “exp.” refers to [34].