Abstract
Temperature-activated transient receptor potential ion channels (thermoTRPs) are polymodal detectors of various stimuli including temperature, voltage, and chemicals. To date, it is not known how TRP channels integrate the action of such disparate stimuli. Identifying specific residues required for channel-activation by distinct stimuli is necessary for understanding overall TRP channel function. TRPV3 is activated by warm temperatures and various chemicals, and is modulated by voltage. One potent activator of TRPV3 is 2-aminoethyl diphenylborinate (2-APB), a synthetic chemical that modulates many TRP channels. In a high-throughput mutagenesis screen of ≈14,000 mutated mouse TRPV3 clones, we found 2 residues (H426 and R696) specifically required for sensitivity of TRPV3 to 2-APB, but not to camphor or voltage. The cytoplasmic N-terminal mutation H426N in human, dog, and frog TRPV3 also effectively abolished 2-APB activation without affecting camphor responses. Interestingly, chicken TRPV3 is weakly sensitive to 2-APB, and the equivalent residue at 426 is an asparagine (N). Mutating this residue to histidine induced 2-APB sensitivity of chicken TRPV3 to levels comparable for other TRPV3 orthologs. The cytoplasmic C-terminal mutation R696K in the TRP box displayed 2-APB specific deficits only in the presence of extracellular calcium, suggesting involvement in gating. TRPV4, a related thermoTRP, is 2-APB insensitive and has variant sequences at both residues identified here. Remarkably, mutating these 2 residues in TRPV4 to TRPV3 sequences (N426H and W737R) was sufficient to induce TRPV3-like 2-APB sensitivity. Therefore, 2-APB activation of TRPV3 is separable from other activation mechanisms, and depends on 2 cytoplasmic residues.
A subset of transient receptor potential (TRP) ion channels have important roles in sensory biology, including pain and thermosensation (1–4). The requirement of several so-called thermoTRPs in thermosensation in vivo has been demonstrated, suggesting that these ion channels have a physiological role as molecular thermometers (5–9). Thresholds of temperature-activation of thermoTRPs are shifted in the presence of chemical agonists, effectively modulating thermosensation (1, 10–12). Several natural and synthetic compounds have been identified as agonists or antagonists for each of the thermoTRPs (13). However, little is known about how these chemicals modulate TRP channel activity.
TRPV3, one of these thermoTRPs, is a nonselective cation channel expressed primarily in mammalian keratinocytes, and is involved in thermosensation (4, 7, 14). Like other thermoTRP channels, TRPV3 is a polymodal detector of temperature (heat) and chemicals. TRPV3 agonists include structurally-related natural compounds such as camphor, thymol, and carvacrol (15, 16). One of the most potent TRPV3 agonists is 2-aminoethyl diphenylborinate (2-APB), a synthetic compound that modulates the activity of many ion channels. It is a common activator of the heat-gated TRPV ion-channels TRPV1, TRPV2, and TRPV3 (17, 18). Besides its action on TRPVs, 2-APB activates TRPA1 and TRPM6, and inhibits TRPM2, TRPM8, TRPC4, TRPC5, and TRPC6 channels (19).
Domains within TRPV3 that confer sensitivity to 2-APB, camphor, or any other chemical are not known. The identity of sites required for response to specific chemical agonists might reveal the location of putative binding sites, and shed light on potential mechanisms of how these ion channels integrate signals from various stimuli. Here, we applied an unbiased random mutation approach to identify residues that are specifically required for TRPV3 chemical sensitivity.
Results
Identification of Mutations Specifically Affecting 2-APB Responses.
We screened a mouse TRPV3 mutant library consisting of ≈14,000 cDNA constructs carrying on average 2.5 mutations per clone generated by error prone PCR. Mutant constructs were transfected into human embryonic kidney (HEK293) cells, and calcium responses evoked by heat (42 °C), 25 μM 2-APB, and 1.75 mM camphor were monitored in a fluorescence imaging plate reader (FLIPR). We recently described the identification of residues specifically required for TRPV3 heat activation (20). Here, we focused on the 2-APB and camphor dataset to identify residues specifically required for chemical responses.
Specifically, we selected clones that showed normal responses for one chemical, but significantly reduced activation by the other (for selection criteria, see Methods). Positive clones were picked, regrown, and full dose-response curves against each stimulus were measured, and EC50 values calculated (see Methods). Seven mutant clones were confirmed as specifically 2-APB deficient, and sequenced. Remarkably, all 7 clones included mutations in 1 of 2 residues. The first, histidine (H) 426, is present in the cytoplasmic N terminus region, between the ankyrin and transmembrane domains. We identified 4 distinct substitutions of H426 in the screen (Table 1). The second, arginine (R) 696 is present within C-terminal cytoplasmic TRP box (IWRLQR), a conserved domain in TRP channels (2, 3). The 3 mutations at this residue have the same closely-related arginine to lysine substitution. All 7 mutations caused severe deficits in 2-APB responses, without affecting camphor responses (Table 1). Although some camphor-deficient clones were also identified, they were relatively more subtle mutations, shifting EC50 values of camphor ≈2-fold or less (data not shown). For this reason, we focused this study on the 2 residues causing specifically >10-fold 2-APB deficiency.
Table 1.
TRPV3 mutants associated with deficiency of 2-APB sensitivity
| Clones | 2-APB EC50, μM | 2-APB nHill | Camphor EC50, mM | Camphor nHill | Mutations | Emax, RFU; 20-mM camphor |
|---|---|---|---|---|---|---|
| WT | 9.0 ± 2.0 | 1.6 | 2.0 ± 0.6 | 2.7 | WT | 61903 |
| C5P2 | >200 | 3.4 | 1.8 ± 0.2 | 3.1 | K617E, R696K | 64345 |
| C11P2 | >200 | 1.9 | 1.7 ± 0.1 | 3.5 | I503T, A543T, R696K | 61741 |
| D2P2 | >200 | 2.9 | 1.8 ± 0.1 | 3.1 | R696K | 52671 |
| E3P2 | >200 | 1.7 | 2.3 ± 0.4 | 2.6 | H426V | 55162 |
| E8P2 | >200 | 3.1 | 1.9 ± 0.1 | 1.9 | H426N | 48852 |
| F3P2 | >200 | 1.7 | 1.8 ± 0.1 | 2.1 | D81V, H426R | 55680 |
| F4P2 | >200 | 2.0 | 1.9 ± 0.1 | 2.5 | H426Q | 49916 |
Initial Electrophysiological Characterization of TRPV3-H426 and TRPV3-R696 Channels.
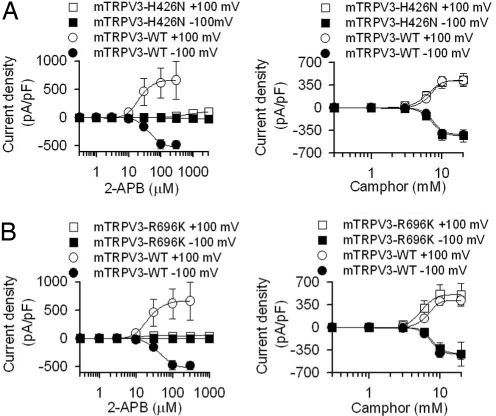
FLIPR is an indirect way of measuring ion channel activity. To confirm our FLIPR results, we used patch clamp technique and measured whole-cell currents of wild-type mouse TRPV3, TRPV3-H426N, and TRPV3-R696K when 2-APB (1 μM to 3 mM) or camphor (0.3 to 20 mM) were individually bath applied from low to high concentrations (Fig. 1 and Fig. S1). In a concentration-dependent manner, 2-APB activated currents in HEK293 cells transfected with wild-type TRPV3 with average EC50 values of 25.9 ± 0.3 (nHill = 2.3) and 36.8 ± 3.6 μM (nHill = 3.3) at +100 and −100 mV, respectively (Fig. 1A; Fig. S1A); 2-APB did not induce measurable currents in HEK293 cells transfected with TRPV3-H426N up to 100 μM. However, concentrations of 2-APB that are saturating for wild-type TRPV3 (0.3 to 3 mM) were able to activate currents in this mutant in a concentration-dependent manner (Fig. 1A; Fig. S1A). Consequently, the TRPV3-H426N showed a >10-fold EC50 shift of 2-APB-dependent activation, and the response never reached saturation. In parallel experiments, camphor also activated currents in HEK293 cells transfected with wild-type TRPV3 in a concentration-dependent manner with EC50 values of 6.7 ± 0.1 (nHill = 7.8) and 7.0 ± 0.1 mM (nHill = 8.4) at +100 and −100 mV, respectively. Importantly, wild-type-like responses to camphor were observed in HEK293 cells transfected with the TRPV3-H426N [EC50 values of 6.3 ± 0.1 (nHill = 7.5) and 6.7 ± 0.1 mM (nHill = 8.1) at +100 and −100 mV, respectively], confirming that camphor activation is unaltered in this mutant (Fig. 1A; Fig. S1B).
Fig. 1.
Electrophysiological characterization of TRPV3-H426N and TRPV3-R696K. (A) Concentration-response curves of 2-APB- and camphor-activated responses in wild-type and H426N mutant reveal specific loss of 2-APB response, but not camphor-evoked response. (B) In R696K mutant, 2-APB-activated response was also specifically affected, whereas camphor-activated responses were similar between wild-type TRPV3 and R696K mutant. For R696K mutant, the acute peak response was used to calculate the EC50 value. Error bars are SEs. For 2-APB responses: n = 17 for wild-type TRPV3; n = 9 for H426N mutant; and n = 6 for R696K mutant. For camphor responses: n = 10 for wild-type TRPV3; n = 5 for H426N mutant; and n = 10 for R696K mutant.
In whole-cell patch-clamp experiments, 2-APB also induced minimal TRPV3-R696K activation. At +100 mV, 2-APB started to activate HEK293 cells expressing this mutant channel at ≈3 μM, and reached saturation at 30 μM, with an EC50 of 10.0 ± 1.3 μM (nHill = 2.1). Higher concentrations of 2-APB (>30 μM) evoked a desensitizing current with an “off-response” after washout of 2-APB, a phenomenon that is described in detail below. However, at −100 mV, 2-APB failed to activate R696K mutant channels even at 1 mM (Fig. 1B; Fig. S1A). The maximal 2-APB response at 1 mM at −100 mV was −2.3 ± 1.4 pA/pF (n = 6), which is significantly smaller (>10-fold) than for wild-type TRPV3 channels (−482.1 ± 69.2 pA/pF at 300 μM, n = 17), suggesting that the maximal channel response evoked by 2-APB is decreased in the TRPV3-R696K mutant. However, camphor activated similar responses to TRPV3- and TRPV3-R696K-transfected HEK293 cells [EC50 values of 5.5 ± 0.2 (nHill = 5.0) and 7.9 ± 0.1 mM (nHill = 5.8) at +100 and −100 mV, respectively] (Fig. 1B; Fig. S1B). Therefore, the initial electrophysiological data confirmed our screening results that TRPV3-H426N and TRPV3-R696K mutants are deficient in 2-APB responsiveness, without affecting overall channel function as tested by their ability to respond to camphor.
We next asked what side-chain properties of H426 and R696 are crucial for TRPV3 activation by 2-APB. We individually mutated positions 426 and 696 to all other amino acids and measured FLIPR dose-response curves for both 2-APB and camphor. Interestingly, all H426 mutants had decreased 2-APB responses with a strong (>10-fold) increase of EC50 values (Table S1). Most of the mutants had normal camphor responses except for the helix-breaking proline substitution, which showed ≈2-fold increase of camphor EC50. The data are consistent with the result of our primary screen that identified 4 different substitutions resulting in 2-APB deficiency at this position. Mutant channels carrying aromatic side-chains F, T, and W did not respond to either camphor or 2-APB. However, most R696 mutants showed reduced 2-APB responses, but also had decreased maximal camphor (20 mM) responses, indicating that these mutations affect overall channel expression or function (Table S2). This result is also consistent with our initial result from the primary screen, where only the lysine substitution was identified to be specifically required for 2-APB at this residue. R696F also showed some 2-APB-specific deficit, although maximal camphor responses were slightly affected. Mutants R696D, R696G, R696N, R696P, R696S, and R696T did not show significant responses to either 2-APB or camphor when compared with pcDNA vector-transfected cells.
Voltage-Dependent Response Remained Intact in 2-APB Mutants.
Voltage-dependence has been shown to have important roles in TRP channel activation and gating (1, 10–12, 21). In the absence of chemical stimulators (for example camphor or 2-APB), voltage steps from −120 to +180 mV (20 mV step) did not activate currents in HEK293 cells transfected with wild-type TRPV3, indicating that TRPV3 (unlike TRPV1 and TRPM8) is not activated by voltage alone at the range tested here (20, 22). However, in the presence of 30 μM 2-APB, a voltage-dependent current was activated (Fig. S2A). As expected, no detectable voltage-modulated current was seen in TRPV3-H426N when treated with 30 μM 2-APB (Fig. S2B). However, 6 mM camphor evoked a voltage-dependent current in HEK293 cells expressing wild-type TRPV3 (V1/2 of 81.3 ± 2.6 mV) (Fig. S2 A and D) and TRPV3-H426N (V1/2 of 81.3 ± 2.5 mV) (Fig. S2 B and D). Similar to TRPV3-H426N, there was no detectable voltage-modulated current in TRPV3-R696K when treated with 30 μM 2-APB (Fig. S2C). However, camphor activated a voltage-dependent current with a V1/2 of 81.1 ± 5.4 mV (Fig. S2 C and D). These results indicate that voltage-modulation is intact in TRPV3-H426N and TRPV3-R696K, and implies that loss of voltage-dependence is unlikely to be the cause of decreased responses to 2-APB in these mutant channels.
Temperature Sensitivity of TRPV3-H426N and TRPV3-R696K.
Temperature sensitivity is a hallmark of TRPV3 channel function (14, 16, 23). Therefore, we asked whether temperature activation was altered in TRPV3-H426N and TRPV3-R696K clones. We measured temperature (25 to 42 °C) activation of HEK293 cells transfected with wild-type TRPV3, TRPV3-H426N, or TRPV3-R696K mutant in a FLIPR assay. Heat responses of TRPV3-H426N were either equal to or slightly larger than wild-type TRPV3 responses (n = 4 experiments), suggesting that 2-APB- and heat-activation can be separated (Fig. S2E; and data not shown). However, heat responses in TRPV3-R696K were much smaller compared with wild type (n = 3 experiments), suggesting that this mutant is not specifically altering 2-APB activation (Fig. S2E).
Calcium-Dependent Desensitization of TRPV3-R696K.
An interesting feature of the 2-APB response in TRPV3-R696K-transfected HEK293 cells is fast desensitization at >30 μM 2-APB and an off-response to 2-APB at high concentrations (Fig. S3). This phenomenon is in contrast to wild-type TRPV3, which sensitizes in response to repetitive/continuous stimulation of chemicals or heat (14, 16). The mechanism underlying TRPV3 sensitization is proposed to be a loss of calcium inhibition of this channel (22). Because desensitization of most other TRP channels (such as TRPV1 and TRPA1) to chemical or temperature stimuli also depends on extracelullar calcium (24, 25), we set out to test whether extracellular calcium has a role in 2-APB-evoked desensitizing responses in TRVP3-R696K. Surprisingly, in the absence of extracelullar calcium, 100 μM 2-APB activated a robust, wild-type-like inward and outward currents in TRPV3-R696K (current density of TRPV3-R696K: 755.7 ± 200.0 pA/pF at +100 mV, −641.2 ± 133.9 pA/pF at −100 mV (n = 5); wild-type TRPV3: 615.0 ± 266.7 pA/pF at +100 mV, and −471.0 ± 83.2 pA/pF at −100 mV, n = 9) (Fig. S4 A and B). This result suggests that replacement of an arginine by a lysine at position 696 in the TRP box of TRPV3 drives the channel into a desensitizing state on 2-APB but not camphor stimulation in a calcium-dependent manner. A putative Ca2+-binding residue in the pore loop domain, aspartate 641, is conserved among all TRPV channels, and is critical to permeation properties of TRP channels (26, 27). Mutation of D641 into asparagine (N) has been shown to reduce ruthenium red block and high affinity extracelullar calcium inhibition of TRPV3 and other TRP channels (17, 22, 26, 27). Therefore, we examined whether the D641N mutation could relieve the TRPV3-R696K clone from calcium-dependent loss of 2-APB response. In a FLIPR assay, the TRPV3-D641N had an EC50 of 2.8 ± 1.3 μM (nHill = 0.7), which is approximately half the value of wild-type TRPV3 (5.7 ± 1.2 μM, nHill = 1.1). The double mutant TRVP3-D641N/R696K had an EC50 of 3.3 ± 1.1 μM (nHill = 1.1), showing that the D641N mutation fully restored the 2-APB response of the TRPV3-R696K. As expected, the camphor response was similar among TRPV3, TRPV3-R696K, TRPV3-D641N, and TRPV3-D641N/R696K (Fig. S4 C and D). These studies demonstrate that TRPV3-R696 is not purely a 2-APB mutation. The deficits observed are calcium-mediated, affecting 2-APB but not camphor responses.
The 2-APB Sensitivity in TRPV3 Orthologs.
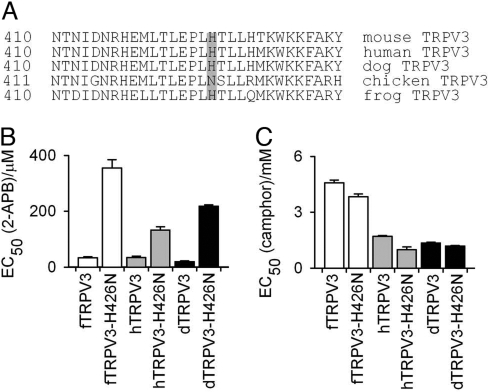
Amino acid conservations and variations among orthologs are useful tools for dissecting structure-function relationships of proteins, including TRP channels (28, 29). Therefore, we asked whether these TRPV3 orthologs have comparable camphor and 2-APB responses. Indeed, human, dog, and frog TRPV3 channels, which have histidines at mouse-equivalent position 426 (Fig. 2A), have similar 2-APB responses when overexpressed in HEK293 cells (although camphor responses in Xenopus tropicalis TRPV3 were decreased compared with wild-type mouse TRPV3) (Fig. 2C). As expected, when H426 in human, dog, and frog was mutated into N, 2-APB concentration-response curves were strongly shifted to the right in each of these TRPV3 orthologs (Fig. 2B). Again, this mutation did not decrease camphor sensitivity when compared with wild-type TRPV3 orthologs (Fig. 2C). On the contrary, both human and frog TRPV3 H426N mutants had slightly higher camphor sensitivity than their wild-type counterparts. These results indicate that the 2-APB activation mechanism of TRPV3 might be conserved throughout different species.
Fig. 2.
A conserved requirement of H426 for 2-APB responses among mammalian and amphibian TRPV3 orthologs. (A) Sequence alignment of mouse, rat, human, dog, frog, and chicken TRPV3. Position 426 is indicated. (B) FLIPR EC50 values of 2-APB (B) and camphor (C) in H426N equivalent mutants of frog, human, and dog TRPV3; fTRPV3 refers to frog TRPV3; hTRPV3 refers to human TRPV3; and dTRPV3 refers to dog TRPV3. Error bars are SEs; n = 5 for each clone.
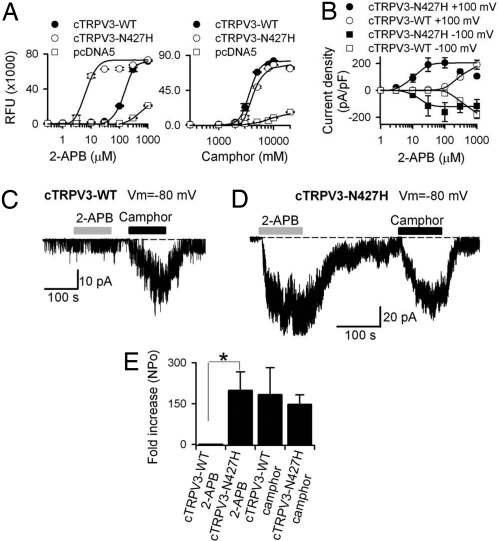
Interestingly, although R696 is conserved in the TRP box of TRPV3 among all species, the position equivalent to mouse 426 in chicken TRPV3 (cTRPV3) is an N (427). As shown above, an H-N substitution at 426 residue in mammalian or amphibian species interferes with proper 2-APB responses (Fig. 2A). Consistent with this residue being important for 2-APB responses, the EC50 of 2-APB in cTRPV3 is 146.8 ± 3.4 μM (nHill = 2.5) as compared with that of 11.5 ± 3.5 μM (nHill = 1.1) for mouse TRPV3. Strikingly, mutating N427 into H shifted the concentration-response curve of 2-APB significantly to the left (EC50 of 6.1 ± 0.9 μM, nHill = 2.3), whereas leaving the EC50 value for camphor unchanged [3.0 ± 0.1 mM (nHill = 5.4) in cTRPV3-N427H vs. 2.6 ± 0.2 mM (nHill = 5.5) in cTRPV3] (Fig. 3A). Whole-cell patch clamp recording confirmed that the 2-APB concentration-response curve was strongly shifted to the left in the chicken TRPV3-N427H as compared with wild-type cTRPV3 at both +100 [cTRPV3 EC50 = 276.1 ± 17.2 μM (nHill = 2.1) versus cTRPV3-N427H mutant EC50 = 10.7 ± 4.9 μM (nHill = 2.1)] and −100 mV [cTRPV3 EC50 = 330.1 ± 22.5 μM (nHill = 2.6) versus cTRPV3-N427H mutant EC50 = 13.0 ± 4.1 μM (nHill = 3.9)] (Fig. 3B). Single-channel recordings revealed almost 200-fold increase of channel openings over the basal level by 25 μM 2-APB in inside-out patches from cTRPV3-N427H-expressing cells (n = 7), whereas no significant changes were observed in inside-out patches from wild-type cTRPV3-expressing HEK293 cells (n = 5). However, 6 mM camphor strongly increased single-channel openings in inside-out patches from HEK293 cells transfected with either wild-type cTRPV3 or cTRPV3-N427H mutant at similar levels (Fig. 3 C–E). Hence, a single point-mutation in chicken TRPV3 specifically increases its sensitivity to 2-APB.
Fig. 3.
N427H mutation restores 2-APB sensitivity of chicken TRPV3. (A) Concentration-response curves of 2-APB and camphor in chicken wild-type TRPV3, cTRPV3-N427H, and pcDNA on FLIPR. Error bars are SEs; n = 6 for each clone. (B) Whole-cell patch-clamp current-density concentration-response curves of 2-APB-activated currents in HEK293 cells transfected with wild-type or N427H mutant chicken TRPV3 at +100 and −100 mV. Error bars are SEs; n = 4 for wild-type chicken TRPV3; and n = 5 for cTRPV3-N427H mutant. (C) Inside-out single channel recordings of wild-type chicken TRPV3 treated with 25 μM 2-APB or 3 mM camphor. (D) Single channel current traces (inside-out configuration) of cTRPV3-N427H channel treated with 25 μM 2-APB or 6 mM camphor. (E) Average fold increase of single channel activities over basal levels evoked by 2-APB or camphor in inside-out patches expressing wild-type cTRP3 or cTRPV3-N427H mutant (*, P < 0.05, Student's t test; n = 4 for wild-type cTRPV3; and n = 5 for cTRPV3-N427H mutant).
The 2-APB Sensitivity in Related ThermoTRPs: TRPV1, TRPV2, and TRPV4.
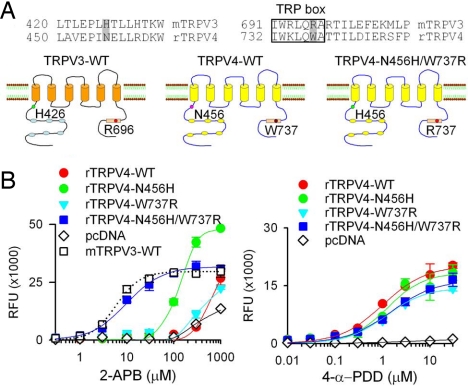
Mammalian TRPV3 is closely related to TRPV1 (39% amino acid homology), TRPV2 (36% homology), and TRPV4 (38% homology). Interestingly, 2-APB is a common agonist of TRPV1, TRPV2, and TRPV3, but not TRPV4 (18). Therefore, we asked whether a common mechanism exists for 2-APB action on these related ion channels. The equivalent position of the mouse TRPV3 His-426 is N in TRPV1 (N419), TRPV2 (N379), and TRPV4 (N456) (Fig. 4A; Fig. S5A). The equivalent position of the mouse TRPV3 R696 in the TRP box is also R (R701) in TRPV1, a conserved lysine (K664) in TRPV2, and a noncharged tryptophan (W737) in TRPV4 (Fig. 4A; Fig. S5A).
Fig. 4.
Mutations of TRPV4 at 2 cytoplasmic residues identified render TRPV4 sensitive to 2-APB. (A) Sequence alignment of the N-terminal region and C-terminal TRP box of mouse TRPV3 and rat TRPV4. Residues required for mouse TRPV3 responses to 2-APB and equivalent residues in TRPV4 are indicated. Diagrams illustrate the positions of the 2 intracellular residues and show a schematic representation of the double mutations in TRPV4. Error bars are SEs; n = 5 for each clone. (B) Concentration-dependent responses to 2-APB and 4-α-PDD in HEK293 cells transfected with wild-type or mutated TRPV4; n = 5 for each clone.
We focused on the N-terminal H residue, which is specifically required for proper 2-APB responses for mammalian and amphibian TRPV3, and an N to H mutation at this residue is sufficient to induce robust 2-APB responses in chicken TRPV3. Wild-type TRPV1 and TRPV2 carry Ns at the equivalent position of TRPV3 H426, but show robust responses to 2-APB. This result raises the possibility that TRPV1 and TRPV2 respond to 2-APB through distinct mechanisms compared with TRPV3. Nevertheless, we tested whether introducing a H at this position specifically affects 2-APB responses in TRPV2 and TRPV1. The TRPV1-N419H mutant had enhanced responses to both 2-APB and capsaicin, indicating a nonspecific effect on channel expression or function (Fig. S5B). The corresponding TRPV2-N379H mutant had similar 2-APB and probenecid (another TRPV2 agonist) responses compared with wild-type TRPV2 (Fig. S5C) (30). These results suggest that the mechanism of 2-APB activation of TRPV2 and TRPV1 is not completely conserved with that of TRPV3 (see Discussion).
We then asked whether the equivalent residues of TRPV3-H426 and R696 are responsible for the lack of 2-APB responses in TRPV4. To address this question, we mutated equivalent amino acid residues at N456 and W737 in TRPV4 to H and R, respectively (Fig. 4A). Wild-type TRPV4 and TRPV4-W737R showed only marginal 2-APB responses compared with vector-transfected controls (Fig. 4B). However, remarkably, HEK293 cells transfected with TRPV4-N456H responded to 2-APB with an EC50 of 131.0 ± 3.3 μM (nHill = 2.4). Also, TRPV4 carrying double mutations N456H/W737R showed even more robust responses to 2-APB, with an EC50 of 10.0 ± 0.8 μM (nHill = 1.2) (close to the value of wild-type mouse TRPV3) (Fig. 4B). Responses to the TRPV4 agonist 4-α-PDD were not significantly different between wild-type TRPV4 and all of the TRPV4 mutants, suggesting that increased 2-APB responses are not due to increased TRPV4 expression level or overall gain of function (Fig. 4B). Recordings in excised inside-out patches expressing TRPV4, TRPV4-N456H, TRPV4-W737R, or TRPV4-N456H/W737R revealed single-channel activity by 150 μM 2-APB in both TRPV4-N456H and TRPV4-N456H/W737R, with the latter showing more robust responses (Fig. S6 B and C). No 2-APB-activated single channel activity was seen in wild-type TRPV4 and TRPV4-W737R (Fig. S6A; and data not shown). These results indicate that differences at the 2 amino acid residues identified in our screen are responsible for the lack of 2-APB sensitivity of TRPV4.
Discussion
Little is known about how each stimulus in TRPV3 is sensed, or how signal integration is achieved. As an initial approach to this question, we have focused on identifying structural elements that are specifically required for sensitivity of thermoTRPs to each individual stimulus (20, 31). Our results show that 2 cytoplasmic residues are each required for activation of TRPV3 by 2-APB, but not by camphor. Positions 426 and 696 emerged in multiple clones from our screen, and subsequent electrophysiological characterization confirmed that mutations H426N and R696K are deficient in 2-APB responses, but not in camphor or voltage responses.
H426 Is Specifically Required for 2-APB Activation of TRPV3.
We show that mutations in H426 dramatically shift the 2-APB EC50 curve, raising the possibility that these mutations might reflect a defect in binding. Importantly, this mutant does not affect other modes of TRPV3 activation (including camphor and heat responses, and voltage modulation). Our experiments on systematically replacing H426 by all other amino acids showed that the 2-APB response cannot be rescued by the positive charge that is also present in R and lysine, or by a residue of similar structure (shape), like phenylalanine. Therefore, the specific requirement of a histidyl side chain at this residue for proper 2-APB responses suggests (although does not prove) direct interaction with the ligand. Interestingly, replacing the chicken TRPV3 N at this position with H is sufficient to induce robust 2-APB activation, consistent with the hypothesis of this N-terminal H being part of a 2-APB binding site. However, it is also possible that chicken TRPV3 binds 2-APB but cannot be efficiently gated. Therefore, a role of the H residue in gating instead of binding cannot be ruled out. Regardless whether H426 constitutes a 2-APB binding site or not, these results show that 2-APB-activation of TRPV3 can be separated from all other agonists. Recently, we have identified a distinct segment close to the TRPV3 pore region that is specifically required for heat but not 2-APB-, camphor-, or voltage-modulation of the channel (20). Together, these findings support a hypothesis in which various modulators of TRPV3 have disparate activation pathways that allosterically couple to gate the channel (1). This hypothesis is also supported by comparing Hill coefficients obtained from dose-response curves of 2-APB and camphor for wild-type TRPV3 channels. Hill coefficients differ almost 2-fold between these 2 agonists, suggesting that the number of agonists required for efficient channel opening or the degree of cooperativity might be larger for camphor than for 2-APB. This disparity is observed in both FLIPR and patch-clamp experiments (although with a consistent shift in FLIPR experiments toward lower Hill coefficients) and consistent throughout all mutants we examined.
Interestingly, the location of H426 is distinct from the location of molecular determinants of TRPV1 sensitivity to capsaicin, and TRPM8 sensitivity to menthol (28, 31, 32). For these botanical chemicals, interaction sites are proposed between transmembrane domains 2 and 4. Instead, the location of cytoplasmic N-terminal H426 residue is reminiscent of the site of action of chemical-activation of TRPA1 (33, 34). Various reactive chemicals such as mustard oil, cinnamaldehyde, and allicin (garlic) activate TRPA1 through cysteine modification, and 3 cytoplasmic cysteines juxtaposing the transmembrane domain in the N terminus of TRPA1 are involved in these responses. Interestingly, allicin was recently shown to activate TRPV1 through a cysteine located at the N-terminal region (35). Therefore, binding of chemicals to this linker region of TRP channels between ankyrin domains and transmembrane helices might cause conformational change and downstream ion channel gating. However, mechanistically, there is no evidence that 2-APB is a covalent modifier, and is likely that 2-APB binds in a reversible lock-and-key fashion.
R696 Within the TRP Box Is Required for Calcium-Dependent 2APB Responses.
The TRP box is a highly conserved hexameric domain in the C-termini of TRP channels, and has been proposed to have a role in subunit multimerization, overall channel gating, and PIP2 binding (1, 36–38). An R residue in the TRP box of TRPV1 (R701) has been implicated in gating the channel to temperature and chemical stimuli (24, 38). Interestingly, mutations in TRPM8 TRP box have been observed to specifically cause loss of menthol activation by influencing the gating properties of the channel (31). In the present study, we found a TRPV3 mutant (R696K) at the equivalent position of TRPV1-R701 in the TRP box that is associated with a loss of 2-APB and heat response, without affecting camphor responses or voltage modulation. Surprisingly, we found out that TRPV3-R696K responded relatively normally to 2-APB in the absence of extracellular calcium. Also, ablating a putative calcium-binding site at the TRPV3 pore, which has been shown to mediate calcium-inhibition, appears to override the 2-APB deficiency of TRPV3-R696K (22). However, because we record wild-type-like camphor responses and observe normal voltage modulation of TRPV3-R696K, it is unlikely that this mutation is causing an overall change in gating efficiency or a general effect of extracellular calcium. However, the majority of mutations at this residue did cause overall channel dysfunction, and, interestingly, most polar side-chain substitutions (glycine, asparagine, serine, and threonine) resulted in nonfunctional channels, suggesting that this residue might be located in a mostly hydrophobic environment. This complex calcium-dependent 2-APB phenotype of the TRPV3-R696, together with the compromised heat-activation of this mutant, argues that this residue has an important role in channel gating in response to some but not all stimuli; 2-APB being an open-channel blocker as proposed by Chung et al. (17) would explain the difference between 2-APB and camphor in the R696K mutant. However, the 2-APB “blocking effect” occurs only in the presence of extracellular calcium, suggesting involvement of additional mechanisms in 2-APB gating in the R696K mutant. The desensitizing property of the R696K mutant prevents accurate measurement of the maximal response of 2-APB in whole-cell patch clamp recording, affecting the accuracy of the EC50 value. It also explains the discrepancy of EC50 values between electrophysiological recording and the FLIPR assay, which measures the concentration of intracellular free calcium ions. Importantly, we have only focused on mutations that specifically affect 2-APB sensitivity; thus, mutants with additional defects in camphor or voltage sensitivity are not characterized. Respective residues might also be required for 2-APB sensitivity in TRPV3 channel and, therefore, revealing additional 2-APB-sensitive elements. However, we cannot rule out a more complicated role in 2-APB binding.
The other possibility is that interaction of 2-APB with the TRP box increases the inhibitory effect of calcium on TRPV3 channel. Although these 2 regions are separated by the cell membrane, it is possible that 2-APB interaction of the TRP box might increase the binding affinity to the pore or transduction efficiency of calcium inhibition of TRPV3 channel. Regardless of the exact role of R696, the finding that both H426 and R696 are critical determinants of 2-APB responses in TRPV3 raises the question whether these cytoplasmic N and C termini interact with each other during resting or activated states of TRPV3.
The 2-APB Responses in TRPV3 Orthologs and Related TRPVs.
The promiscuous TRP channel modulator 2-APB can function either as an agonist or antagonist of many TRP channels within 3 subfamilies (19). In this work, we explored whether the molecular determinants of 2-APB sensitivity of mouse TRPV3 is shared among TRPV3 orthologs, and related TRPVs. TRPV3 orthologs are present among mammals, birds, and clawed frogs, but not in bony fishes and invertebrates. We found that the N-terminal H426 residue is conserved in human, mouse, dog, and frog, and is required for proper 2-APB responses in all these species. Interestingly, chicken TRPV3 responds poorly to 2-APB and has an N at the equivalent residue. We show that substituting a H at this position is sufficient to induce robust 2-APB responses in chicken TRPV3. The change in chicken TRPV3 sequence at this residue compared with mammalian and frog TRPV3 sequences raises the possibility that birds have specifically lost this H residue, and consequently have lost the ability to respond to 2-APB (28). However, 2-APB is a synthetic compound, and whether a structurally-similar endogenous or botanical molecule exists that acts as a natural TRPV3 agonist is unknown.
The mechanism of 2-APB sensitivity of TRPV1 and TRPV2 appears to be at least partly distinct from TRPV3, as both of these 2-APB-sensitive channels do not have histidines at the equivalent position of mTRPV3-H426. However, remarkably, we show that changing 2 TRPV4 residues to TRPV3-like sequences is sufficient to induce 2-APB sensitivity in TRPV4, suggesting that shared elements are required for 2-APB activation between these 2 related channels.
Methods
Mutant Library.
The method of generating a random mutant library and high-throughput screening for TRPV3 mutants is described previously (20, 31). Error prone PCR (Diversify PCR Random Mutagenesis Kit, Clontech) was performed on the cloned mouse TRPV3 gene and subsequently the PCR product was cloned into the pcDNA5-FRT mammalian expression vector.
Mutagenesis.
All site-directed mutants were generated by QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene). All clones were verified by DNA sequencing.
Cell-Culture/Transfection/FLIPR.
The methods of cell culture, DNA transfection and FLIPR are described previously (20, 31). For each mutant clone fluorescence increase evoked by 2-APB or camphor was measured in quadruplicate and averaged. Responses were normalized to wild-type TRPV3-transfected control wells (100%). The normalized 2-APB response was subtracted from the normalized camphor response. Clones having values of >60% were considered as 2-APB-specific mutants in the primary screening. For concentration-response curves, maximal responses were calculated after baseline subtraction. Averages and SDs were calculated from 3 wells, responses normalized to unity and fitted by a Hill equation. Cut-off values for hit-confirmation were obtained from calculating average values and SD of pooled data from controls from all plates. Mutants that satisfied the following 3 criteria were picked: (i) 2-APB EC50 values are >3 SDs from the average of wild-type TRPV3; (ii) camphor EC50 values are <1 SD from the average of wild-type TRPV3; and (iii) camphor-evoked maximal response at 20 mM (Emax) are <2.5 SDs from the average of wild-type TRPV3.
Cloning of X. tropicalis, Canine, and Chicken TRPV3.
Cloning of X. tropicalis TRPV3 was described previously (20). Total RNAs from canine and chicken brain tissues were purchased from Zyagen. First strand cDNA was prepared from the total RNA by using SuperScript II reverse transcriptase (Invitrogen). PCR was peformed by using the following primers: for chicken TRPV3, sense: 5′-ATG ATT AAA GAT AAC AAA GAA GTT GTT CCA CTA ATG GGC-3′ and antisense: 5′-CTA AAG AGA AGT TTC AGG CAA ATC ATC CG-3′; for canine TRPV3, sense: 5′-ATG AAT GCC CAC CCC AAG GAG ATG GTG CCC CTC ATG GGC AGA AGA G-3′, antisense: 5′-CTA CAC TGA AGT TTC CGG AAA TTC ATC-3′. The PCR products were cloned into a pCR2.1-TOPO vector (Invitrogen) and subcloned into pcDNA5-FRT vector by using KpnI/NotI sites.
Electrophysiology.
The method of whole cell patch clamp recording is described previously (20, 31). Conductance-voltage (GV) curves were obtained from plateau currents of voltage-step protocols by calculating the conductance for each data point in a single experiment separately and normalizing it to the maximal response. For quantitative single channel analysis the Pulse files were converted to Axon Binary Files, analyzed, and plotted by using Clampfit 9.2 (Molecular Devices).
Supplementary Material
Acknowledgments.
We thank Myleen Medina, Anthony Marelli, Jia Zhang, and Tony Orth for preparing miniprep DNA; Michael Caterina (Johns Hopkins School of Medicine, Baltimore) for providing rat TRPV1 plasmid DNA; and Tim Jegla and A.P. laboratory members for helpful discussion.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812209106/DCSupplemental.
References
- 1.Latorre R, Brauchi S, Orta G, Zaelzer C, Vargas G. ThermoTRP channels as modular proteins with allosteric gating. Cell Calcium. 2007;42:427–438. doi: 10.1016/j.ceca.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 4.Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R64–76. doi: 10.1152/ajpregu.00446.2006. [DOI] [PubMed] [Google Scholar]
- 6.Dhaka A, et al. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 7.Moqrich A, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 8.Colburn RW, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Bautista DM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 10.Matta JA, Ahern GP. Voltage is a partial activator of thermo-sensitive TRP channels. J Physiol. 2007 doi: 10.1113/jphysiol.2007.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilius B, et al. Gating of TRP channels: A voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voets T, et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 13.Macpherson LJ, et al. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Peier AM, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 15.Vogt-Eisele AK, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 17.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu HZ, et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 19.Clapham DE. SnapShot: Mammalian TRP channels. Cell. 2007;129:220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Grandl J, et al. Pore region of TRPV3 ion channel is specifically required for heat activation. Nat Neurosci. 2008;11:1007–1013. doi: 10.1038/nn.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao R, et al. Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J Biol Chem. 2008;283:6162–6174. doi: 10.1074/jbc.M706535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith GD, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 24.Novakova-Tousova K, et al. Functional changes in the vanilloid receptor subtype 1 channel during and after acute desensitization. Neuroscience. 2007;149:144–154. doi: 10.1016/j.neuroscience.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Martinez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem. 2000;275:32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- 27.Voets T, et al. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- 28.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 29.Saito S, Shingai R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics. 2006;27:219–230. doi: 10.1152/physiolgenomics.00322.2005. [DOI] [PubMed] [Google Scholar]
- 30.Bang S, Kim KY, Yoo S, Lee SH, Hwang SW. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci Lett. 2007;425:120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Bandell M, et al. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
- 32.Voets T, Owsianik G, Janssens A, Talavera K, Nilius B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat Chem Biol. 2007;3:174–182. doi: 10.1038/nchembio862. [DOI] [PubMed] [Google Scholar]
- 33.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 35.Salazar H, et al. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Sanz N, et al. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci. 2004;24:5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Sanz N, et al. A role of the transient receptor potential domain of vanilloid receptor I in channel gating. J Neurosci. 2007;27:11641–11650. doi: 10.1523/JNEUROSCI.2457-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valente P, et al. Identification of molecular determinants of channel gating in the transient receptor potential box of vanilloid receptor I. FASEB J. 2008;22:3298–3309. doi: 10.1096/fj.08-107425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.