Table 1.
Intermolecular Diels–Alder Reactions Involving Oxazolidinone Dienophiles.
| Entry | Reacting partners | Conditions | Major product | exo/endoa | Yield |
|---|---|---|---|---|---|
| 1b | 1a + 3* | 1.4 equiv. Me2AlCl, CH2Cl2, −40 °C | 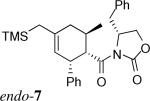 |
1:5 | 50% |
| 2c | 1b + 3* | 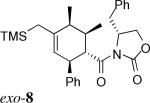 |
> 20:1 | 58% | |
| 3d | 1c + 3* | 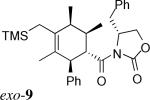 |
> 20:1 | 65% | |
| 4e | 2a + 4 |
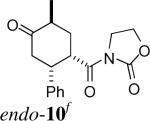 f f
|
1:20 | 78% | |
| 5 | 2a + 3 | 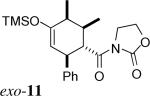 |
> 20:1 | 62% | |
| 6 | 2b + 3 | 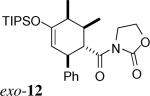 |
> 20:1 | 89% | |
| 7 | 2c + 3 | 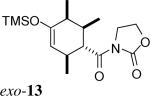 |
> 20:1 | 80% | |
| 8 | 2d + 3* | 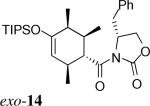 |
> 20:1 | 48% | |
| 9 | 1a + 5 | 0.2−0.3 equiv. Me2AlCl, CH2Cl2, RT | 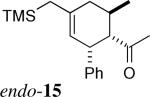 |
1:4 | 15% exo 62% endo |
| 10c | 1b + 6 | 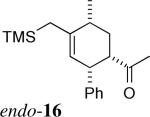 |
1:10 | 4% exo 40% endo | |
| 11c | 1b + 5 | 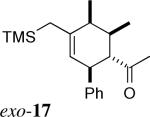 |
11: (1:1.5:0.4)g | 78% exo |
By 400 MHz 1H NMR of the product mixture after aqueous workup.
ref. 20.
1b used as an inseparable isomeric mixture with (Z,E):(E,E) = 3:1. Reaction of only the major isomer was observed unless otherwise stated.
1c used as a mixture with (Z,E):(E,E) = 5:1. Only reaction from the major isomer was observed.
At −100 °C.
Product of protodesilylation of the primary, endo cycloadduct, epimerized at C5, was isolated after chromatographic purification over silica gel.
Four sets of signals were observed by 1H NMR of the crude mixture. Two of the adducts were assigned as exo-17 and its C-5 epimer. The other two, minor adducts were tentatively assigned as the corresponding endo isomers.
