Table 3.
Diels-Alder Reactions of 5 and Dienes
| entry | diene | product | conditiona | yield(%)b |
|---|---|---|---|---|
| 1 | 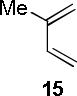 |
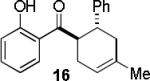 |
A B |
97c 67c |
| 2 | 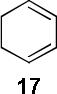 |
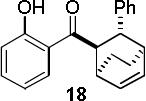 |
A B |
97d 65d |
| 3 | 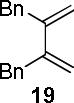 |
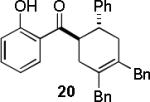 |
A | 99 |
| 4 | 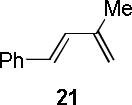 |
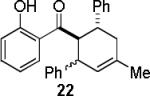 |
A | 97c,e |
| 5 | 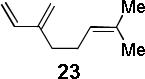 |
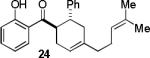 |
A | 96c |
| 6 | 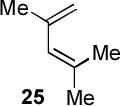 |
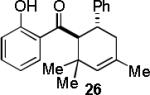 |
A | 55c |
Condition A: 10/10/60/10 mol% CoI2/8/ZnI2/Bu4NBH4, 40°C; condition B: 60/10 mol% ZnI2/Bu4NBH4, 40°C, see Supporting Information.
Isolated yields.
Single regioisomer.
Single endo isomer.
1.5:1 exo/endo ratio.
