Figure 2.
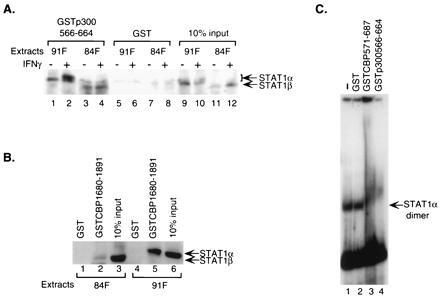
Endogenous Stat1 homodimers interact with CBP/p300. (A) Both unphosphorylated and phosphorylated cellular Stat1α and β interact with the CREB-binding domain of CBP/p300. GST-fusion proteins of CBP/p300 bound on glutathione-Sepharose beads were incubated with nuclear extracts from untreated or IFN-γ-treated U3A cells deficient for endogenous Stat1 and stably expressing FLAG-tagged Stat1α or β. The specifically bound Stat1 proteins were analyzed by Western blotting using an anti-FLAG antibody. 84F, extracts from cells expressing FLAG-tagged Stat1β; 91F, extracts from cells expressing FLAG-tagged Stat1α. (B) Interaction between cellular Stat1α and the E1A-binding domain of CBP requires the carboxyl terminus of Stat1α. Whole-cell extracts from treated cells stably expressing FLAG-tagged Stat1α or β as in A were incubated with GST-fusion proteins and the resulting bound Stat1 analyzed as above. (C) The CREB-binding domain of CBP/p300 interacts with Stat1α dimer bound to DNA. Nuclear extracts from IFN-γ-treated U3A cells reconstituted with FLAG-tagged Stat1α were incubated with a 32P-labeled Stat1-DNA-binding site before being exposed to various purified GST-fusion proteins, and the resulting DNA–protein complexes were analyzed by EMSA.
