Figure 2.
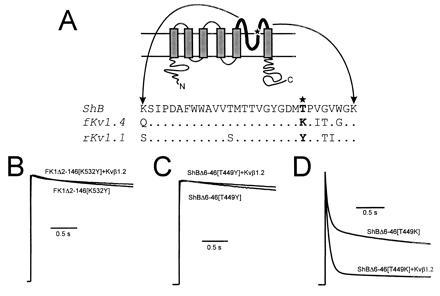
Kvβ1.2 influence on the rate of C-type inactivation rate in the presence of pore mouth mutations that affect C-type inactivation. (A Upper) Schematic representation of a voltage gated K+ channel 6-membrane spanning domain structure. The bold line represents the pore forming domain, with the star showing the approximate position of the pore mouth mutations. (Bottom) Alignments of the amino acid sequence of the pore regions from ShB (24), ferret Kv1.4 (15), and rat Kv1.1 (25). Mutations that alter the rate of C-type inactivation were constructed at position 449 in Shaker and 532 in fKv1.4 (★). (B) Coexpression of Kvβ1.2 with FK1Δ2-146[K532Y] had no effect on inactivation rate (P > 0.25). (C) Coexpression of Kvβ1.2 with ShBΔ6-46[T449Y] also had no effect on inactivation rate (P > 0.25). (D) ShBΔ6-46[T449K] mutation greatly increased the rate of C-type inactivation. Total inactivation measured at 100 ms increased 1.4 fold (Table 1; P < 0.05) in response to Kvβ1.2 coexpression. All current traces shown are in response to a depolarizing pulse to +50 mV from a holding potential of −90 mV. Peak current values measured at +50 mV in 2 mM [K+]o were 5.6 ± 1.6 μA for FK1Δ2-146[K532Y], 21 ± 4 μA for ShBΔ2-46[T449Y], 3.4 ± 1.2 μA for FK1Δ2-146[K532Y] plus Kvβ1.2, 28 ± 8 μA for ShBΔ2-46[T449Y] plus Kvβ1.2, 17 ± 1 μA for ShBΔ2-46[T449K], and 19 ± 3 μA for ShBΔ2-46[T449K] plus Kvβ1.2.
