Figure 3.
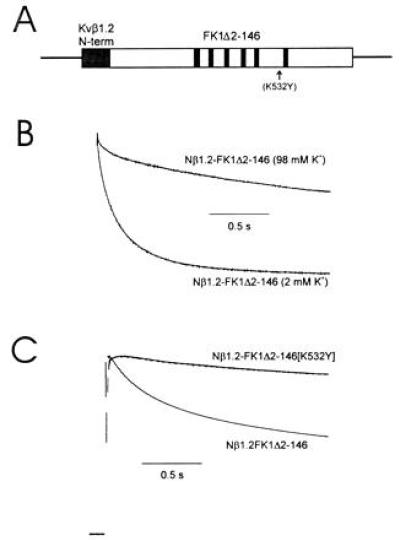
The N-terminal domain of Kvβ1.2 increases the rate of C-type inactivation of FK1. (A) Diagram of the Kvβ1.2–FK1 chimeric construct. Amino acids 1–79 were spliced to the N terminus of FK1Δ2-146 (B and C) or FK1Δ2-146[532Y] (C). (B) The chimeric construct between Kvβ1.2 and FK1 showed an increased rate of inactivation which was sensitive to an increase of [K+]o. (C) Inactivation of the chimeric construct was greatly slowed by a mutation in the pore region (K532Y) that largely removed C-type inactivation. Current traces shown are in response to a depolarizing pulse to +50 mV from a holding potential of −90 mV. Currents were normalized to peak current for purposes of comparison of inactivation rates. Peak current values measured at +50 mV in 2 mM [K+]o were 11 ± 3.5 μA for the chimera and 21 ± 4 μA for the mutant chimera.
