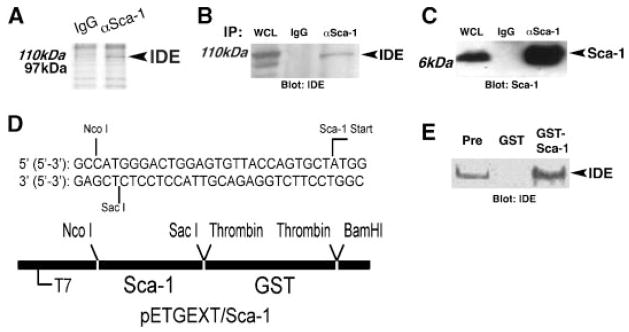
Fig. 4.
Sca-1 associates with Insulin Degrading Enzyme. A: Lysates from C2C12 myoblasts grown under differentiation conditions for 24 h were immunoprecipitated with anti-Sca-1 antibody (αSca-1) versus isotype control (IgG), and separated by SDS–polyacrylamide gel electrophoresis. Coomassie Brilliant blue staining identified aSca-1-dependent band at~110kDa protein, which was identified as IDE by LC–MS/MS analysis. B:Sca-1 was immunoprecipitated from differentiating C2C12 myoblasts, and immunoblot analysis showed that IDE (arrow) specifically co-precipitates with Sca-1 (αSca-1), compared to control immunoprecipitation with isotype control antibody (IgG). WCL, whole cell lysate. C: Immunoblot analysis as in (B) demonstrated Sca-1 immunoprecipitation. D: Details of GST-Sca-1 fusion construct in pETGEXT designed to exclude ER retention signal and GPI-anchor addition sequences. E: Empty vector (GST) and GST-Sca-1 were expressed in bacteria, purified, and used to pull down complexes from lysates of C2C12 myoblasts after 24 h of differentiation. Immunoblot analysis identified IDE in the pre-pulldown lysate (Pre), in lysate incubated with GST-Sca-1, but not in lysate incubated with GST alone.