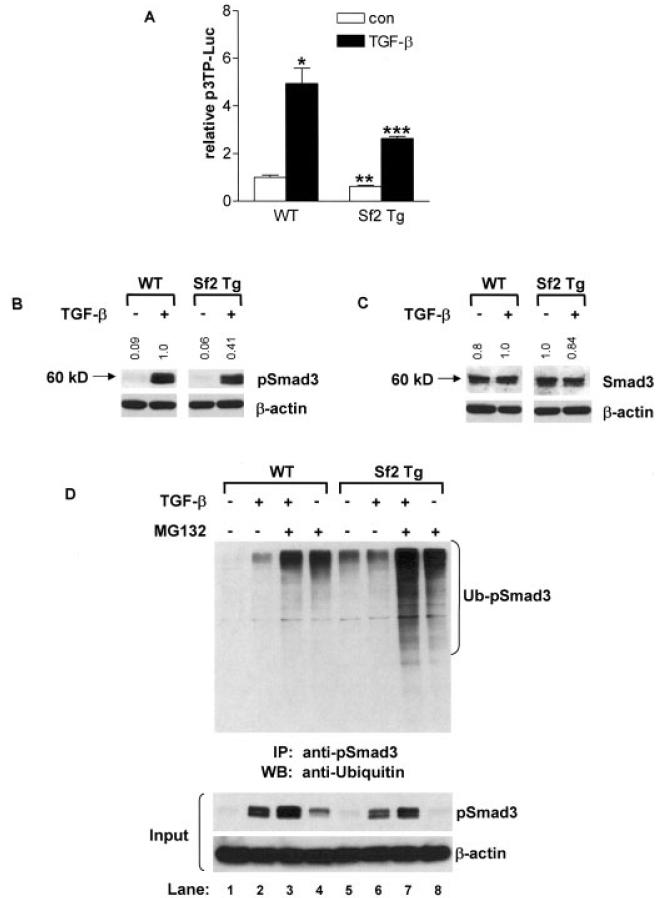
Figure 6.
Inhibition of transforming growth factor β (TGFβ) signaling in neonatal sternal chondrocytes overexpressing Smurf2. Sternal chondrocytes were isolated from 3-day-old wild-type (WT) and Col2a1–Smurf2 (Col2a1-Sf2)–transgenic (Sf2 Tg) mice. A, Sternal chondrocytes were cotransfected with the p3TP-luciferase (p3TP-Luc) reporter and the SV40 Renilla luciferase plasmid. Cells were treated with 5 ng/ml of TGFβ1 or with vehicle (control [con]), and after 48 hours, cells were lysed, and extracts were evaluated for luciferase activity. Values are the mean and SEM of 3 mice per group. * = P < 0.05 and ** = P < 0.05 versus vehicle-treated WT chondrocytes; *** = P < 0.05 versus TGFβ1-treated WT chondrocytes, by analysis of variance. B and C, Sternal chondrocytes were treated with 5 ng/ml of TGFβ1 for 30 minutes, and total cellular protein was extracted, and the expression of pSmad3, Smad3, and β-actin proteins was evaluated by Western blotting. Fold changes in protein levels are indicated at the top of each representative blot (from 3 repeats), after controlling for protein loading (versus β-actin), using National Institutes of Health ImageJ software. Molecular weight markers are shown at the left. D, Using an anti-pSmad3 antibody, immunoprecipitation (IP) was performed on extracts of sternal chondrocytes that had been pretreated for 3 hours with 10 μM MG132 or vehicle, followed by a 30-minute treatment with 5 ng/ml of TGFβ1. The immunoprecipitated proteins were analyzed by Western blotting (WB) using a polyclonal anti-ubiquitin antibody. Western analysis of input samples was performed using anti-pSmad3 or anti–β-actin antibodies. Blots are representative of a series of 3 repeats. Ub-pSmad3 = ubiquitinated pSmad3.