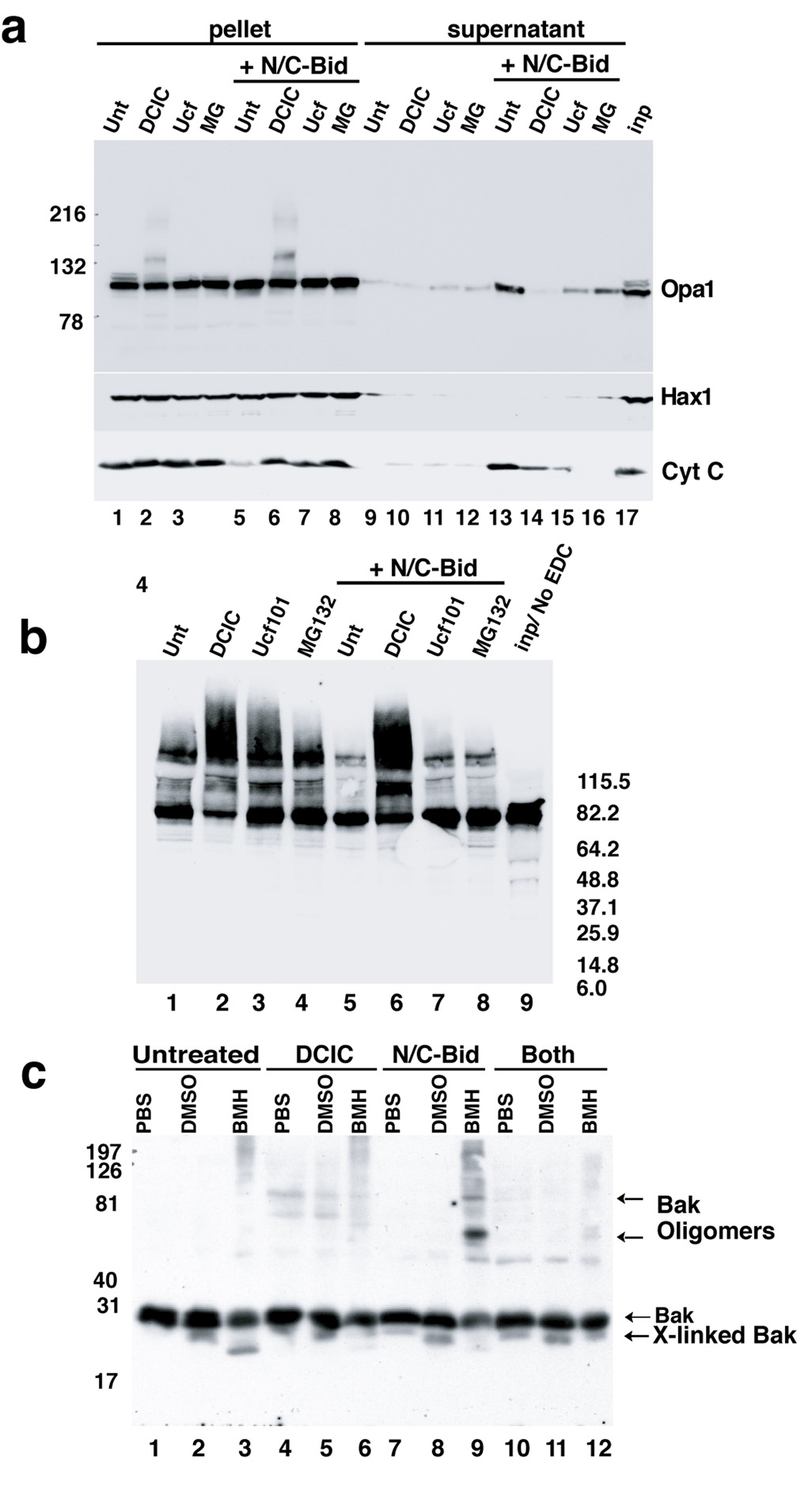
Fig. 3. N/C-Bid-induced disassembly of the OPA1 complex in the absence of MOMP.
(a) Inhibition of cytochrome c release by three serine protease inhibitors. Mitochondria were suspended at 5 mg/ml in the trehalose buffer either with (lanes 5–8, 9–12 and 17) or without (lane 1–4 and 13–16) 10 nM N/C-Bid. Either 100 µM DCIC (lanes 2, 6, 10 and14), 25 µM MG132 (lanes 3, 7, 11 and 15), or 50 µM Ucf101 (lanes 4, 8, 12 and 16) was added to samples, followed by an incubation for 30 min at 37° C. Mitochondrial pellets were separated on 4–12% gradient gels and Western blotted with anti-Opa1 (upper panel), anti-Hax1 (middle panel) and anti-cytochrome c antibody (bottom panel). Note the altered mobility of OPA1 in lanes 2 and 6 in the upper panel. (b) Ucf101 and MG132 did not block disassembly of the OPA1 complex. Mitochondrial pellets from (a) were suspended in 10 mM EDC in PBS and incubated for 45 min at 37° C. Cross-linked samples were separated by 4–12% gradient gel and Western blotted with anti-OPA1 antibody. (c) MOMP inhibitors blocked N/C-Bid-induced Bak oligomerization. Mitochondria were treated with either 100 µM DCIC (lanes 4–6), 10 nM N/C-Bid (lanes 7–9) or both (lanes 10–12) or left untreated (lanes 1–3) for 30 minutes at 37° C. Pellets were suspended either in PBS (lanes 1, 4, 7 and 10), 10% DMSO in PBS (lanes 2, 5, 8 and 11) or 10 mM BMH (Pierce) in PBS (lanes 3, 6, 9 and 12) and incubated for 45 min at 37° C. Samples were separated by 12% SDS-PAGE and Western blotted with anti-Bak antibody. The locations of Bak, cross-linked Bak and Bak oligomer are indicated. Bcl-xL, Ucf101 and MG132 also blocked Bak oligomerization (Suppl. Fig. 1).