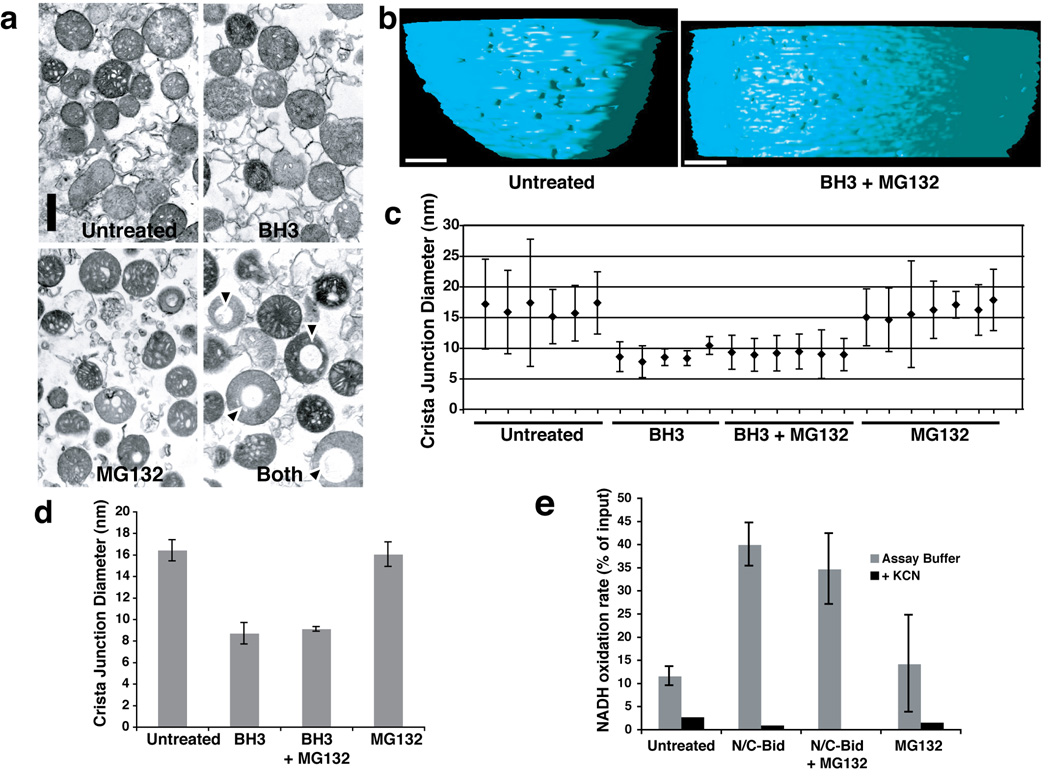
Fig. 4. Crista junction remodeling induced by a BH3 domain peptide was not blocked by MG132.
Isolated mitochondria were suspended in trehalose buffer at 5 mg/ml and incubated for 15 min at 37° C in the presence of 20 µM Bid BH3 peptide, 100 µM MG132, both agents, or no addition. a. Bid BH3 peptide did not induce gross mitochondrial remodeling. Mitochondrial pellets were analyzed by transmission electron microscopy. Note that mitochondria treated with both the BH3 peptide and MG132 often contained large vacuoles, but otherwise showed no remodeling of cristae. b. Electron tomographic images of crista junctions in untreated mitochondria and mitochondria treated with BH3 + MG132. The volumes were segmented and surface-rendered. The inner boundary membrane for two conditions (untreated and MG132+N/C-Bid) is shown in side view. Note the size difference in crista junction openings. c, d. Crista junction sizes were reduced by treatment with BH3 peptide, and this was unaffected by MG132. c. The graph represents mean and standard deviation of cristae junction sizes from untreated (sample 1–6), BH3-treated (samples 7–11), BH3+MG132-treated (samples 12–17) and MG132-treated (samples 18–24) mitochondria. Scales in nm. d. The average of cristae junction sizes from 4 different groups of mitochondria above were analyzed and presented. Error bars represent standard deviation. e. N/C-Bid increased cytochrome c accessibility to the outer membrane, and MG132 failed to inhibit this increase. The rate of NADH oxidation in the presence of complex I and II inhibitors were measured for mitochondria treated either with MG132, N/C-Bid, or both, or left untreated. The y-axis represents the rate of NADH loss calculated as % of input per 10 s.