Abstract
Background
Between the ages of 45 and 65 years, the prevalence of cardiovascular disease is significantly lower in women compared with men. Circulating bone marrow-derived endothelial progenitor cells (EPCs) play an important role in vascular repair. Reduced EPC number is predictive of more cardiovascular events. It is currently unknown whether there is a sex-difference in EPC number in middle-aged adults.
Objective
We tested the hypothesis that circulating EPC number is higher in middle-aged women than men.
Methods
Peripheral blood samples were collected from 58 sedentary adults, 29 men (57 ± 1 yr) and 29 women (58 ± 1 yr). Mononuclear cells were isolated and fluorescence-activated cell sorting (FACS) analysis of cells negative for CD45 was performed for those positive for CD34, and triple positive for CD34, VEGFR-2, and CD133 according to the recommendations of the International Society for Hematotherapy and Graft Engineering.
Results
The number of CD45-/CD34+ and CD45-/CD34+/ VEGFR-2+/CD133+ were not significantly different between women and men (0.055 ± 0.006% vs 0.069 ± 0.008% and 0.0013 ± 0.0003% vs 0.0018 ± 0.0004%, respectively).
Conclusions
These results demonstrate no sex-difference in EPC number in middle-age adults. Therefore, it is unlikely that differences in EPC number contribute to the gender-related differences in the prevalence of cardiovascular events in this population.
Keywords: Endothelial Progenitor Cells, Gender, Endothelium
INTRODUCTION
Cardiovascular disease remains the leading cause of death in both men and women. However, between the ages of 45 and 65, men have a significantly higher risk of myocardial infarction and stroke than women in the absence of a gender-related difference in the prevalence of traditional cardiovascular disease risk factors such as hypertension, tobacco use, obesity, diabetes, sedentary behavior and hyperlipidemia.1 It is now apparent that vascular endothelial dysfunction is a critical prelude to all major cardiovascular diseases, including myocardial infarction and stroke. Endothelial dysfunction occurs when vascular repair mechanisms are unable to keep pace with on-going vascular injury (i.e. from pathologic conditions such as hypertension, hyperlipidemia, etc.).2
Bone marrow-derived endothelial progenitor cells (EPCs) play an integral role in the maintenance of vascular health. EPC-mediated vascular repair is associated with normalization of endothelial function and restoration of blood flow at the site of injury.3 Clinical interest in circulating EPCs has increased in the wake of recent studies indicating that reductions in EPC levels are an excellent predictor of cardiovascular events4-7 and a surrogate marker for vascular function.4 Moreover, increases in circulating EPC number in response to interventions such exercise, smoking cessation and statin therapy are thought to contribute to their cardiovascular benefit.8, 9
While mechanisms underlying the observed gender-difference in clinical cardiovascular events in middle-aged adults are not fully elucidated, we recently demonstrated important gender-differences in contributors to vascular health. Middle-aged men demonstrate lower endothelial tissue-type plasminogen activator (t-PA) release compared to women.10 Impairment in the capacity of the endothelium to release t-PA has been directly linked to increased atheromatous burden and increased coronary atherothrombosis.11 Given the important link between circulating EPCs, endothelial function and vascular disease it is possible that differences in EPC number may also contribute to the gender-related difference in cardiovascular events in middle-aged adults.
It is currently unknown whether circulating EPC number differs between middle-aged women and men. Lower circulating levels of EPCs may contribute to the higher incidence of cardiovascular morbidity and mortality in middle-aged men compared with women. Accordingly, we tested the hypothesis that the number of circulating EPCs is lower in middle-aged men compared with women.
METHODS
Subjects
Peripheral blood samples were collected from 58 healthy, sedentary middle-aged adults, 29 men and 29 women. All subjects were nonobese (body mass index ≤30 kg/m2), normotensive, nonsmokers, nonmedicated, and free of overt cardiovascular, metabolic, and hematologic diseases as assessed by medical history, electrocardiograms obtained during exercise and at rest, and fasting blood chemistries. The women were ≥1year postmenopausal (range 1 to 25 years) and had never taken or discontinued use of hormone replacement therapy ≥1 year before the start of the study. Framingham risk score was calculated as previously described 12. All subjects provided written informed consent and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the University of Colorado at Boulder human research committee.
EPC Isolation and Number
Circulating EPC number was determined by fluorescence-activated cell sorting (FACS) analysis following guidelines recommended by the International Society for Hematotherapy and Graft Engineering.13 Briefly, peripheral blood mononuclear cells (PBMNCs) were isolated by Ficoll density-gradient centrifugation (Histopaque 1077, Sigma, St. Louis, Missouri), washed, and resuspended in growth medium (Medium 199, Gibco, Grand Island, New York), supplemented with 20% fetal calf serum, penicillin (100U/ml), and streptomycin (100 mg/ml). Endothelial phenotype of these cells was confirmed in a subset of samples by immunofluorescent staining for the uptake of DiI-ac-LDL (Biomedical Technologies Inc., Stoughton, MA, USA) and expression of von Willebrand factor (Dako, Glostrup, Denmark), VE-cadherin, CD31 and VEGFR-2 (Invitrogen, Carlsbad, CA, USA).
Cells (2.0× 106) were incubated at 4°C for 30 minutes with antibodies against PC7-conjugated CD45, FITC-conjugated CD34, PE-conjugated VEGFR-2, and APC-conjugated CD133. Nonviable cells were excluded with propidium iodide. Samples were gated first for lack of expression of CD45 then analyzed for those events positive for CD34 and triple positive for CD34, VEGFR-2 (also previously identified as KDR or Flk-1) and CD1337, 14, 15 and presented as percent of total viable cells. All samples were analyzed using a FC500 flow cytometer (Beckman Coulter, Fullerton CA) and the data analyzed using CXP software.
Recent data demonstrate that CD34+ cells not expressing the common leukocyte antigen (CD45-) were found to yield endothelial colony-forming cells, while those expressing CD45 demonstrated hematopoietic progenitor properties. 16 Therefore, in accordance with previous studies 14, 17, 18, we identified putative EPCs as those without expression of CD45 while still expressing the additional markers as outlined above.
Statistical Analysis
Group differences in subject characteristics and FACS data were determined by analysis of variance (ANOVA). Relations between variables of interest were assessed by Pearson’s correlation coefficient and linear regression analysis. Based on the magnitude of the differences that were observed in EPC number between groups, post hoc power calculations indicated that the sample size was sufficient to detect significant main effects. Data are reported as mean±SEM. Statistical significance was set at P<0.05.
RESULTS
Subject Characteristics
Selected subject characteristics are presented Table 1. Although none of the subjects were obese, the men demonstrated significantly higher body mass and waist circumference and lower percent body fat compared with the women. Other than high-density lipoprotein cholesterol, there were no significant gender-related differences in metabolic or hematologic variables. There were no differences in Framingham risk score between men and women, but the estimated 10-year coronary heart disease risk was significantly higher in men than in women. We observed no relation between risk score or estimated risk and EPC number.
Table 1.
Selected subject characteristics.
| Variable | Men (n=29) |
Women (n=29) |
|---|---|---|
| Age, y | 57±1 | 58±1 |
| Years postmenopausal, yr | ----- | 8±1 |
| Body Mass, kg | 84.9±2.3 | 70.4±2.7* |
| Body Fat, % | 27.8±1.3 | 38.6±1.3* |
| Body mass index, kg/m2 | 27.1±0.7 | 26.0±0.9 |
| Waist circumference, cm | 97.3±1.8 | 83.6±2.8* |
| Systolic blood pressure, mmHg | 125±2 | 120±2 |
| Diastolic blood pressure, mmHg | 77±1 | 73±1* |
| Total cholesterol, mmol/L | 5.1±0.1 | 5.4±0.1 |
| HDL cholesterol, mmol/L | 1.1±0.1 | 1.7±0.1* |
| LDL cholesterol, mmol/L | 3.3±0.1 | 3.3±0.2 |
| Triglycerides, mmol/L | 1.4±0.1 | 1.2±0.1 |
| Glucose, mmol/L | 5.2±0.1 | 5.1±0.1 |
| Insulin, pmol/L | 44.4±5.1 | 42.0±5.1 |
| WBC, 1000/μL | 5.2±0.2 | 5.1±0.2 |
| Mononuclear cells, % | 40.5±1.2 | 42.2±1.4 |
| Framingham risk score | 5.0±0.4 | 4.0±0.6 |
| Estimated 10-yr CHD risk, % | 9.6±0.7 | 4.9±0.5* |
Values are mean±SEM. HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC, White Blood Cell Count; CHD, coronary heart disease;
P<0.05 vs men.
EPC Number
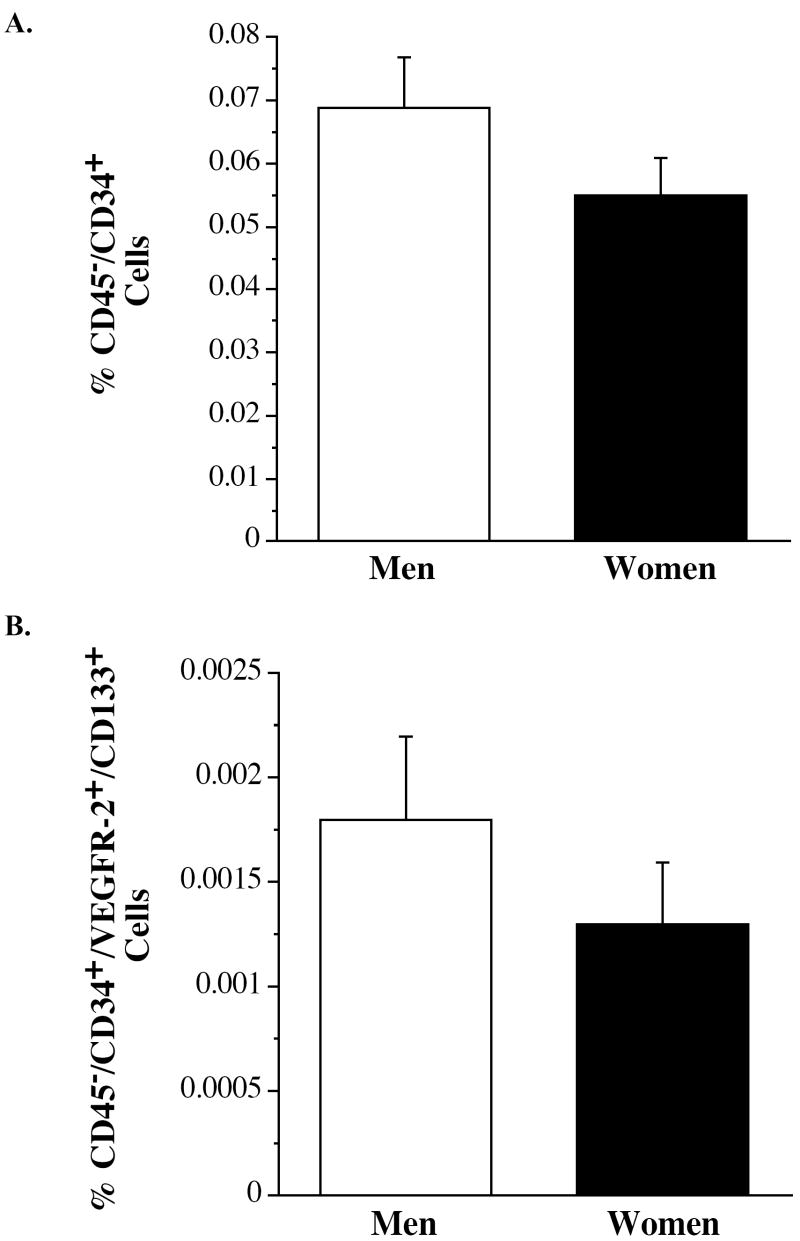
Mononuclear cells were first gated for CD45- to exclude leukocytes and decrease the likelihood of false-positive EPC identification. To discover whether the overall number of these hematopoietic precursor cells is different between men and women the number of CD34 + cells was determined. Surprisingly, there was no significant difference in CD45-/CD34+ hematopoietic precursor cells between middle-aged women and men (0.055 ± 0.006% vs 0.069 ± 0.008%, Figure 1a). In addition, we observed no significant gender-related difference in circulating putative EPCs defined as CD45-/CD34+/VEGFR-2+/CD133+ cells. The percentage of cells staining triple positive for CD34, VEGFR-2 and CD133 were comparable between middle-aged women and men (0.0013 ± 0.0003% vs 0.0018 ± 0.0004%, P=0.40, Figure 1b).
Figure 1.
Percentage of CD45-/CD34+ (panel A) and CD45-/CD34+/VEGFR-2+/CD133+ (panel B) cells in middle-aged men and women.
DISCUSSION
The novel finding of the present study is that EPC number is not different between middle-aged women and men. Although circulating EPC number is predictive of cardiovascular morbidity and mortality in a number of clinical populations4, 5, 19, our study suggests that EPC number may not contribute to differences in the cardiovascular event rate between middle-aged men and women. Moreover, it is unlikely that differences in EPC number contribute to the previously documented gender-difference in endothelial function.10 To our knowledge, this is the first study to investigate gender-differences in the number of circulating CD45-/CD34+/VEGFR-2+/CD133+ cells in healthy adults.
Recently Fadini, et. al. investigated the influence of gender and estrogen on CD34+/VEGFR-2+/CD133+ cell number.20 While they did not demonstrate any gender difference in their cell population, the expression of CD45, a hematopoietic lineage-specific antigen was not evaluated. Case et. al. demonstrated that CD45 expression was an effective measure to differentiate hematopoietic progenitors from endothelial progenitors16, a critical distinction when evaluating the vascular progenitor cell. Thus, while Fadini et. al. demonstrated no gender difference in a heterogenous progenitor population containing both hematopoetic and endothelial precursors, their results cannot exclude the possibility of a gender difference specifically in the endothelial precursor population.16 Importantly, it is the cells with endothelial lineage that have been associated with cardiovascular outcomes. The findings of the current study demonstrate no gender difference in a specific EPC population.
Cell surface antigens identify various stages of progenitor cells. CD34 is expressed by all hematopoietic stem cells and is lost as hematopoietic cells differentiate.21 CD34+ cells are involved in a number of cardiovascular processes. For example, in the myocardium they home to sites of ischemia, enhance neovascularization, and contribute to improved cardiac function.22 Indeed, intramyocardial transplantation of CD34+ cells into rats with myocardial infarction has been shown to improve capillary density, fractional shortening and regional contractility.23 The number of CD34+ cells is highly susceptible to cardiovascular risk factors. For instance, CD34-positive cells are diminished with cigarette smoking, increase with smoking cessation, and decline after subjects resume smoking.14 A seminal finding of the present study is that gender does not appear to affect circulating CD34+ cells. We observed similar numbers of CD34-positive cells in healthy middle-aged, non-obese women and men.
While there is some debate currently in the literature regarding which antigenic profile best identifies progenitor cells with the potential to repair the endothelium 24, triple positive cells identified by the dual expression of CD133, a surface antigen that identifies more immature progenitor cells than CD34 alone, and VEGFR-2, an indicator of early endothelial differentiation, in the CD45-/CD34+ population are generally accepted as being progenitors of endothelial lineage.16, 25 The number of circulating CD45-/CD34+/ VEGFR-2+/CD133+ cells has been shown to be 50% to 70% lower in patients with coronary artery disease or Eisenmenger syndrome and individuals who smoke cigarettes, respectively.7, 14, 18 Moreover, these cells are inversely-related and independently predictive of carotid intimal medial thickness, an indicator of early subclinical atherosclerosis.26 Both single (CD45-/CD34+) and triple positive (CD45-/CD34+/VEGFR-2+/CD133+) cells are mobilized following myocardial infarction, presumably to assist with myocardial repair and vascular regeneration.17 Importantly, a reduction in EPCs that express the VEGFR-2 antigen predict future cardiovascular events 5 and death from cardiovascular causes.6
One important limitation of the current study is that only EPC number demonstrated by FACS analysis was evaluated. It is becoming more evident in the literature that EPC function ex vivo is an independent marker of vascular health. Thus, differences in EPC functional characteristics may contribute to differences in cardiovascular events amongst middle aged men and women. Indeed, we have previously demonstrated a significant gender-related difference in two aspects of EPC function, colony-forming capacity and migration, which have been linked to cardiovascular risk. 27 Taken together, these finding suggest that greater EPC function, but not number, may contribute to the lower cardiovascular risk in middle-aged women compared to men.
In conclusion, it is well established that impairment of the vascular endothelium is an important precursor to atherosclerosis and cardiovascular events. Endothelial dysfunction occurs when repeated endothelial damage, such as from traditional cardiovascular risk factors, overwhelms the endogenous vascular repair mechanisms. We previously demonstrated that middle-aged women have higher endothelial t-PA production compared with men, indicating endothelial dysfunction in men corresponding to the age of gender-discordance in cardiovascular event rates. There is increasing evidence that EPCs play a pivotal role in vascular repair and endothelial replacement. Given the body of literature documenting the relation between EPCs and clinical cardiovascular events, we were surprised to find no gender-dependent difference in EPC number in middle-aged adults. In spite of the predictive value of EPC number in other populations, there was no difference in EPC number between men and women in the age range where there is a substantial gender-difference in the prevalence of cardiovascular events. Therefore, a difference in EPC number does not appear to be a contributing factor to the gender-related disparity in the cardiovascular event rate in the middle-aged adult population.
Acknowledgments
This study was supported by awards HL080212, HL076434, HL077450 and MO1 RR00051 from the National Institutes of Health, Bethesda, Maryland and award 0555678Z from the American Heart Association, Dallas, Texas. We thank all the subjects who participated in the study and Yoli Casas, MS for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46(1):7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 3.Fujiyama S, Amano K, Uehira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93(10):980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 4.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 6.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 7.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 8.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 9.Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103(24):2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 10.Stauffer BL, Hoetzer GL, Van Guilder GP, Smith DT, Desouza CA. Gender differences in endothelial tissue-type plasminogen activator release in middle-aged adults. J Am Coll Cardiol. 2005;45(9):1547–1549. doi: 10.1016/j.jacc.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Newby DE, McLeod AL, Uren NG, et al. Impaired coronary tissue plasminogen activator release is associated with coronary atherosclerosis and cigarette smoking: direct link between endothelial dysfunction and atherothrombosis. Circulation. 2001;103(15):1936–1941. doi: 10.1161/01.cir.103.15.1936. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 14.Kondo T, Hayashi M, Takeshita K, et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24(8):1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 15.Distler JH, Allanore Y, Avouac J, et al. EUSTAR statement and recommendations on endothelial precursor cells. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.091918. [DOI] [PubMed] [Google Scholar]
- 16.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35(7):1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Numaguchi Y, Sone T, Okumura K, et al. The impact of the capability of circulating progenitor cell to differentiate on myocardial salvage in patients with primary acute myocardial infarction. Circulation. 2006;114(1 Suppl):I114–119. doi: 10.1161/CIRCULATIONAHA.105.000588. [DOI] [PubMed] [Google Scholar]
- 18.Diller GP, van Eijl S, Okonko DO, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117(23):3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 19.Quyyumi AA, Hill JM. Circulating endothelial progenitor cells as novel biological determinants of vascular function and risk. Can J Cardiol. 2004;20 Suppl B:44B–48B. [PubMed] [Google Scholar]
- 20.Fadini GP, de Kreutzenberg S, Albiero M, et al. Gender differences in endothelial progenitor cells and cardiovascular risk profile: the role of female estrogens. Arterioscler Thromb Vasc Biol. 2008;28(5):997–1004. doi: 10.1161/ATVBAHA.107.159558. [DOI] [PubMed] [Google Scholar]
- 21.Katz FE, Tindle R, Sutherland DR, Greaves MF. Identification of a membrane glycoprotein associated with haemopoietic progenitor cells. Leuk Res. 1985;9(2):191–198. doi: 10.1016/0145-2126(85)90082-7. [DOI] [PubMed] [Google Scholar]
- 22.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto A, Iwasaki H, Kusano K, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114(20):2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 24.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106(5):1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 25.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: isolation and characterization. Trends Cardiovasc Med. 2003;13(5):201–206. doi: 10.1016/s1050-1738(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 26.Fadini GP, Coracina A, Baesso I, et al. Peripheral blood CD34+KDR+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37(9):2277–2282. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- 27.Hoetzer GL, MacEneaney OJ, Irmiger HM, et al. Gender differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am J Cardiol. 2007;99(1):46–48. doi: 10.1016/j.amjcard.2006.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]