Fig. 2.
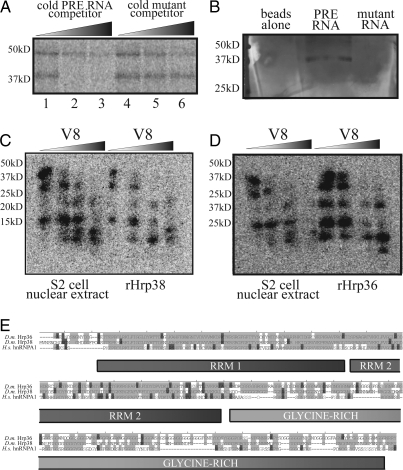
Hrp38 and Hrp36 interact with the PRE. (A) A 40-kDa protein in Drosophila Kc cell nuclear extract specifically cross-links to radiolabeled PRE RNA upon UV irradiation. Radiolabeled PRE (30 fmol) was cross-linked to proteins in nuclear extract after incubation with 300 fmol (10×; lanes 1 and 4), 3 pmol (100×; lanes 2 and 5), or 30 pmol (1,000×; lanes 3 and 6) competitor RNA. (B) A ≈40-kDa band was the most abundant selected from Drosophila S2 cell nuclear extract using immobilized wild-type PRE RNA. Mass spectrometric analysis revealed the major protein in this band to be Hrp38 and a minor protein to be Hrp36. (C) Partial proteolysis pattern of the PRE-cross-linked 40-kDa protein from extract and recombinant Hrp38. (D) Partial proteolysis pattern of recombinant Hrp36 is highly similar to that of the 40-kDa protein and Hrp38. In A–D the positions of marker proteins are indicated on the left. (E) Sequence alignment of Drosophila (D.m.) Hrp36 and Hrp38, which are ≈80% identical, and human (H.s.) hnRNP A1. Light gray indicates identical amino acids and dark gray shows similar amino acids.