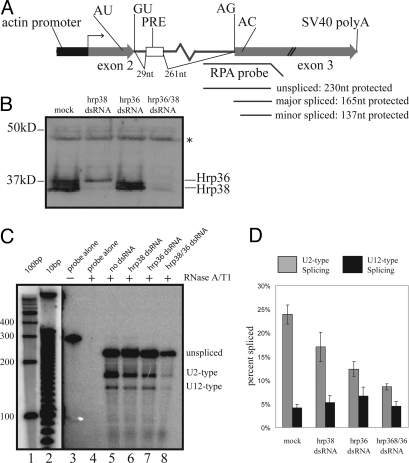
Fig. 3.
dsRNA knockdown of Hrp36 and/or Hrp38 results in lower U2-type splicing efficiency, but does not dramatically influence U12-type splicing efficiency. (A) In vivo splicing substrate used to assess twintron splicing. 5′ and 3′ splice sites of U2-type (GU/AG) and U12-type (AU/AC) introns are indicated, with regions of the antisense probe protected by unspliced precursor, major-spliced product, or minor-spliced product shown. (B) Western blot analysis of lysate from mock- or dsRNA-treated cells indicates reduction in protein expression. The antibody was rabbit polyclonal anti-Hrp38 (see Materials and Methods), which cross-reacts with Hrp36. Asterisk indicates a lysate protein that nonspecifically cross-reacts with the antiserum and therefore serves as a loading control. Hrp36 migrates slower than Hrp38 not only because of intrinsic differences in molecular mass (Hrp36 is ≈39.5kDa, and the 2 major Hrp38 isoforms are 38 and 39 kDa) but perhaps posttranslational modifications of Hrp36 as well (52). (C) RPA shows that the levels of U2-type splicing decrease upon dsRNA-mediated knockdown of Hrp38 and Hrp36 individually (lanes 6 and 7, respectively) or together (lane 8). The yield of total RNA harvested from S2 cells treated with both anti-hrp36 and anti-hrp38 dsRNA was considerably lower than that obtained from untreated or singly treated cells, resulting in lower signals of unspliced, U2-type spliced, and U12-type spliced products in the ensuing RPA (compare lanes 5–7 with lane 8). (D) Quantitation of U2- versus U12-type spliced products. Values are the average of 5 independent experiments, with SDs shown.