Abstract
Collagen content and cross-linking are believed to be major determinants of tendon structural integrity and function. Sex and chronic resistance training have been shown to alter tendon function and may also alter the key structural features of tendon. Patellar tendon biopsies were taken from untrained men [n = 8, 1 repetition maximum (RM) = 53 ± 3 kg], untrained women (n = 8, 1 RM = 29 ± 2 kg), and resistance-trained (10 ± 1 yr of training) men (n = 8, 1 RM = 71 ± 6 kg). Biopsies were analyzed for dry mass, collagen content, and collagen cross-linking (hydroxylysylpyridinoline). We hypothesized that these elements of tendon structure would be lower in women than men, whereas chronic resistance training would increase these parameters in men. Tendon dry mass was significantly lower in women than men (343 ± 5 vs. 376 ± 8 μg dry mass/mg tendon wet wt, P < 0.01) and was not influenced by chronic resistance training (P > 0.05). The lower tendon dry mass in women tended to reduce (P = 0.08) collagen content per tendon wet weight. Collagen content of the tendon dry mass was not influenced by sex or resistance training (P > 0.05). Similarly, cross-linking of collagen was unaltered (P > 0.05) by sex or training. Although sex alters the water content of patellar tendon tissue, any changes in tendon function with sex or chronic resistance training in men do not appear to be explained by alterations in collagen content or cross-linking of collagen within the dry mass component of the tendon.
Keywords: human tendon, water content, hydroxyproline, hydroxylysylpyridinoline
tendons are the fibroelastic structures that connect muscle to bone and convey muscular force (5, 22, 23). The tensile strength of tendon tissue is estimated to be 50–100 N/mm2 (5), a key feature as tendons are subjected daily to high forces. The composition of tendon tissue influences its strength and integrity, playing a key role in force transmission through the muscle-tendon complex (23). Animal and human cadaver studies indicate that tendon tissue is ∼55–70% water (5, 23), with the remaining dry mass containing the extracellular matrix and tendon cells (5, 22). Collagen is the main protein component of tendon tissue, comprising ∼65–75% of the dry mass (5, 22, 23). Collagen molecules are joined into fibrils and fibers by lysine-based cross-links [hydroxylysylpyridinoline (HP) and lysylpyridinoline (LP)] (7, 22, 23, 30). Tendon dry mass, collagen content, and cross-links have been shown to impact tendon strength and mechanical properties (22, 23, 30, 52).
Tendon tissue is dynamic, highly metabolic, and quite responsive to exercise (23, 24, 29, 34). Tendons should therefore adapt in a positive manner to long-term loading, becoming more damage resistant and ensuring optimal muscular force transmission (23). In humans, acute exercise stimulates collagen synthesis (29, 39), and chronic exercise appears to increase tendon strength via structural adaptations at the whole tendon and the molecular level. With training, tendon cross-sectional area (CSA) is increased (24, 25, 35, 46), tendon mechanical properties are improved (27, 44), and there is a net positive balance of collagen (28). Although the impact of exercise training on collagen content and cross-linking in human tendon tissue is unknown, animal data show increased collagen content with chronic training (37, 54), accompanied by increased CSA and altered tendon mechanics (54). Taken together, these data indicate that the acute and chronic response of the tendon is to increase collagen content and/or the degree of collagen cross-linking, thereby improving tendon tissue integrity and force transmission.
Incidence of training-related tendon pathologies is significantly greater in women than men (10, 17, 21), and women <30 yr of age may be at the greatest risk for overuse injuries (31). The cause of this sex-related disparity is unknown, but female tendon tissue may have less collagen and cross-linking than male tendon tissue. Lower collagen content and less collagen cross-linking in women may also contribute to the potentially inferior tendon mechanics in women, namely, increased tendon strain and stress with decreased stiffness (26, 33, 53). Animal data show significantly less collagen per tendon weight in female than male tendons (36). In humans, tendon collagen synthesis is depressed in women compared with men at rest and after acute exercise (38). Additionally, the characteristic increase in tendon size that occurs in men with training does not occur in women (33, 53). These sex-related differences may be due to the biology of tendon cells, which have sex hormone receptors (17), as collagen protein synthesis after exercise is blunted by increased estradiol (33).
We determined dry mass, collagen content, and collagen cross-linking using patellar tendon biopsy samples from three groups (21–35 yr of age): recreationally active men, recreationally active women, and chronically resistance-trained men (≥3 yr of consistent training). We hypothesized that these tendon structural characteristics would be lower in women than men and would be increased by chronic resistance training in men.
MATERIALS AND METHODS
Subjects
Three groups of healthy young adults (21–35 yr of age) were recruited to participate in the study: untrained men (n = 8), untrained women (n = 8), and chronically resistance-trained men (n = 8). Subject characteristics are presented in Table 1. Before enrollment into the study, subjects gave written consent for participation and completed a detailed health history questionnaire and interview regarding their health and exercise-training history. All individuals were apparently healthy and had no history of tendon pathologies. The study was approved by the Institutional Review Board of Ball State University.
Table 1.
Subject characteristics
| Group | Age, yr | Height, m | Weight, kg | BMI, kg/m2 | 1 RM, kg | Years RT |
|---|---|---|---|---|---|---|
| M | 25±1 | 1.78±0.02 | 79±2 | 25±1 | 53±3 | |
| W | 23±2 | 1.69±0.02‡ | 67±3‡ | 24±1 | 29±2‡ | |
| RTM | 24±2 | 1.82±0.02 | 90±8* | 30±2† | 71±6* | 10±1 |
Values are means ± SE (n = 8 per group). M, untrained men; W, women; RTM, resistance-trained men; BMI, body mass index; 1 RM, 1 repetition maximum (unilateral leg extension); Years RT, years of consistent resistance training (≥4 days/wk, 1 h/day). Significantly greater than men:
P < 0.05;
P = 0.08. Significantly less than men:
P < 0.05. No significant differences were found between groups for age.
All subjects in the untrained groups were recreationally to moderately active; i.e., the subjects did not perform aerobic or resistance exercise on a regular basis and did not participate in organized sports. At the time of the study, three of the eight resistance-trained men were engaged in competitive Olympic weightlifting, and one other subject had an extensive history of participation in the sport. Minimum requirements for resistance-trained men were as follows: they were engaged in resistance training, including lower-body training, ≥4 days/wk at the time of the study, and they had trained in this fashion for ≥3 yr. These parameters were based on previous research demonstrating significant musculotendinous improvement with training programs of equal or lesser duration (24, 25, 27, 42, 44). Actual years of subjects' resistance training far exceeded these requirements (Table 1).
Experimental Protocol
Prebiopsy controls.
Participants visited the laboratory before their experimental trial for anthropometric (height and weight) measurements and for their pretrial instructions, premeasured water, and evening meal. Participants refrained from exercise training or vigorous physical activity for the 3 days before the experimental trial and from alcohol and caffeine consumption 24 h before the trial. The evening meal (Ensure Plus, Ross, Columbus, OH) provided 50% of the subjects' estimated daily caloric need (16) and standardized the composition, amount, sodium content, and timing of the final prebiopsy meal. Subjects also followed guidelines (11, 18, 43) to standardize the influence of hydration status on tissue fluid balance: ad libitum water consumption during the 3 days before their experimental trial, 1 liter of water between 4 PM and bedtime on the evening before their trial, and a standardized amount of water (7 ml/kg body wt) 1 h before arrival at the laboratory. For standardization of the impact of estrogen and progesterone levels on water balance (11, 48, 49), all women completed their trial during days 3–7 of their menstrual cycle as reported by the subject. On the morning of their trial, all subjects reported to the laboratory for a tendon biopsy of the left patellar tendon, followed by strength testing of the right leg.
Tendon biopsy.
Subjects reported to the laboratory on the morning of their trial and were supine for ≥45 min (average ∼50 min) before the biopsy was obtained from the patellar tendon of the left leg (39). After administration of local anesthetic (1% lidocaine HCl), tissue was obtained using a Magnum biopsy instrument and a 14-gauge needle (MG1522 and MN1410, Bard, Covington, GA). The tendon sample was immediately inspected under ×10–20 magnification, and tendon tissue was identified by its banded appearance and consistency relative to any peritendinous tissue. The sample was then cleaned of any peritendinous tissue and frozen in liquid nitrogen within 1 min to minimize water loss. Samples were stored in liquid nitrogen (−190°C) until determination of tissue wet and dry weight, collagen content, and collagen cross-links. A small number (n = 3) of peritendinous samples were also analyzed for these parameters to determine the impact of contamination of the sample tissue if not fully cleared via microscope dissection.
Strength testing.
Each subject underwent strength testing to determine the unilateral one repetition maximum (1-RM) of the knee extensors of the right leg (Table 1). Before the 1-RM measurement, each subject performed a warm-up consisting of 5 min on a stationary cycle followed by additional low-load resistance repetitions. For the determination of 1 RM, each subject was instructed to lift and lower the applied weight. Additional weight was added after completion of a single repetition. After a standard 2-min rest period, the subject repeated the lift, and the addition of weight and rest between attempts continued until full extension could no longer be achieved. The maximum weight lifted to full extension was considered the 1 RM.
Tendon Tissue Analysis
Tendon tissue dry mass.
Tissue wet weight (∼3.5 mg) was obtained via precision microbalance (AD-2Z Autobalance, Perkin-Elmer, Wellesley, MA) at −35°C. Samples were placed in vented cryovials, freeze-dried for ∼36 h (Flexi-Dry Freezer-Dryer, FTS Industries, New York, NY), and reweighed at −35°C. This second measure served as the dry mass of the tissue (∼1.3 mg). Tendon wet weight and dry mass were used to calculate the relative amount of dry mass per tendon wet weight (μg dry mass/tendon wet wt).
Hydroxyproline analysis for collagen content.
Tendon collagen content was determined via measurement of hydroxyproline (HYP), one of three predominant amino acids in collagen (6, 12). HYP was quantified by HPLC and fluorometric detection (1100 Series, Agilent Technologies, Wilmington, DE) via the precolumn derivatization method previously described (19, 20) with modifications for human tendon. Briefly, tendon biopsy samples were placed in 6 M HCl (2 mg tendon wet wt/ml HCl) and hydrolyzed for 24 h at 100°C. A 250-μl aliquot of hydrolysate was removed and added to 750 μl of 6 M HCl; then the diluted hydrolysate was neutralized with 1 ml of 6 M NaOH and titrated to pH 8–13. HYP (model 56250, Sigma, St. Louis, MO) standards (1, 10, 50, 100, 150, 200, and 250 μM HYP) were prepared in water.
A 900-μl aliquot of each sample/standard was transferred to a 12 × 75 mm borosilicate tube, and 200 μl of borate buffer (0.7 M boric acid, pH 9.5), followed by 100 μl of OPA solution [50 mg of o-phthalaldehyde dissolved in 974 μl of acetonitrile (ACN) and 26 mg of β-mercaptoethanol], 100 μl of iodoacetamide reagent (140 mg/ml iodoacetamide in ACN), and 300 μl of 5 mM FMOC solution (9-fluorenylmethylchloroformate in acetone), were added to the sample/standard. Tubes were vortexed, and 60 s elapsed after the addition of each reagent.
Ethyl ether (2 ml) was added to each tube, which was then tightly capped and vigorously shaken for 30 s to wash the contents. The organic layer was discarded, and the wash was repeated twice for a total of three washes. Derivatized samples and standards were injected onto the HPLC (1100 Series, Agilent Technologies) via an autosampler (5 μl) every 40 min with an intervening wash step. All samples and standards were run in triplicate. HYP separation was achieved via an XTerra RP 18, 5-μm, 250 × 4.6 mm column (Waters, Milford, MA) using an isocratic mobile phase [65% acetic acid-35% acetonitrile (3% glacial acetic acid and sodium acetate buffered to 4.3)] at a flow rate of 1.0 ml/min. Peaks were monitored at 260 nm excitation/316 nm emission with a gain of 8 and integrated with chromatography software (ChemStation, Agilent Technologies). HYP concentration was determined and used to calculate collagen content, as previously described (6, 20).
HP and LP cross-link content.
Molecular cross-linking of collagen via lysine-based cross-links, purported to be the most prevalent cross-linking pathway in collagen (7, 30), was assessed by measurement of HP via HPLC and fluorometric detection (1100 Series, Agilent Technologies), as previously described (1, 2) with modifications for human tendon. From stored hydrolysate, 500 μl of prepared sample were aliquoted into 16 × 100 mm glass tubes and evaporated to dryness (Savant Speed-Vac sample concentrator, Global Medical Instrumentation, Ramsey, MN). Lyophilized samples were reconstituted with 200 μl of cross-link sample buffer [0.5% heptafluorobutyric acid (HFBA) in 10% acetonitrile], and then 20 μl of 10−5 M pyridoxine (dissolved in HFBA-ACN sample buffer) were added to serve as the internal standard.
A single solution containing both HP and LP (Pyd/Dpd Internal Calibrator, 8004, Quidel, Santa Clara, CA) was used to generate separate standard curves for each type of cross-link. Standards were prepared in HFBA-ACN sample buffer using the HP/LP standard and the internal standard pyridoxine to a final volume of 220 μl in the following concentrations: 0.19, 0.74, 1.54, 2.28, 3.70, and 5.19 μM HP and 0.08, 0.33, 0.70, 1.03, 1.67, and 2.34 μM LP.
Samples and standards were injected onto the HPLC (1100 Series, Agilent Technologies) and eluted as described by Bank et al. (1, 2) with modifications for human tendon. HP and LP separation was achieved with an XTerra RP 18, 5-μm, 250 mm × 4.6 mm column (Waters) that had been equilibrated with 0.13% HFBA in 22% methanol (mobile phase A). Cross-links were then eluted with a 1.0 ml/min flow rate with mobile phase A followed by a secondary mobile phase using 0.1% HFBA in 75% ACN (mobile phase B). Fluorescence was monitored at 295 nm excitation/395 nm emission at a gain of 14, and peaks were integrated with chromatography software (ChemStation, Agilent Technologies). Chromatographs indicated that LP was present in only some human tendon samples at very low levels (not reliably reproducible); therefore, we do not report values for LP.
Statistics
For each variable (subject characteristics, tissue dry mass, collagen content, and collagen cross-links), a two-tailed t-test was used for group comparisons. Group comparisons were as follows: 1) men vs. women and 2) men vs. resistance-trained men. Significance was accepted at P < 0.05. Values are means ± SE.
RESULTS
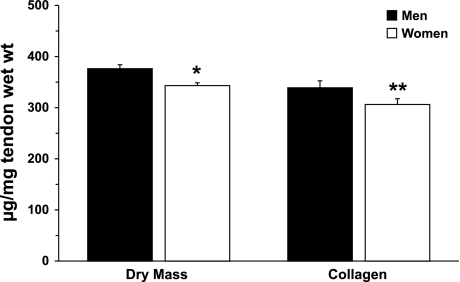
Men had a significantly greater amount of tendon dry mass than women (376 ± 8 vs. 343 ± 5 μg dry mass/mg tendon wet wt, P < 0.01). The lower dry mass in women reduced (P = 0.08) collagen content per wet weight tendon (339 ± 14 and 306 ± 11 μg collagen/mg tendon wet wt in men and women, respectively). Figure 1 compares the in vivo patellar tendon composition of men and women: tissue dry mass per tendon wet weight and collagen content per tendon wet weight.
Fig. 1.
In vivo tendon dry mass and collagen content in men and women. Expression of dry mass and collagen content per tendon wet weight demonstrates the functional amounts of these elements in whole tendon. *P < 0.01; **P = 0.08 vs. men.
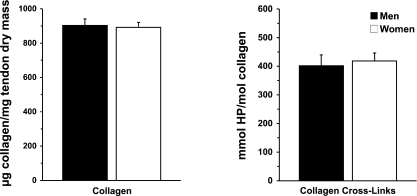
When normalized to tendon dry mass, collagen content was comparable in men and women (903 ± 38 and 892 ± 29 μg collagen/mg tendon dry mass, respectively, P > 0.05). HP cross-linking of collagen was similarly unaltered by sex (401 ± 47 and 418 ± 35 mmol HP/mol collagen in men and women, respectively, P > 0.05). Figure 2 compares the dry tendon material composition of untrained men and women: collagen content per tendon dry mass and HP cross-linking to collagen.
Fig. 2.
Dry tendon collagen content and collagen cross-linking in men and women. Normalization of collagen to dry mass demonstrates amount of collagen in nonwater material of tendon tissue. Hydroxylysylpyridinoline (HP) cross-links relative to collagen reveal number of cross-links per collagen in tendon tissue. Taken together, these 2 variables indicate quality of biosynthesized tendon tissue, independent of tissue water content. No differences (P > 0.05) were observed between men and women.
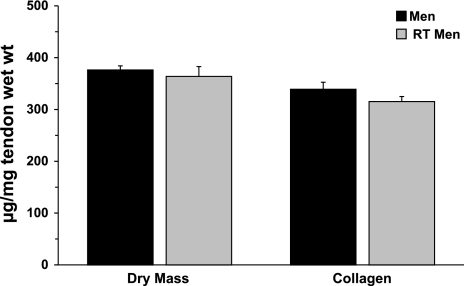
Chronically resistance-trained men (10 ± 1 yr of resistance training) demonstrated no higher levels of either parameter than untrained men. Tendon dry mass was unaltered with chronic resistance training (376 ± 8 and 364 ± 20 μg tendon dry mass/mg tendon wet wt in untrained and resistance-trained men, respectively). Collagen content normalized to tendon wet weight was unchanged by training (339 ± 14 and 315 ± 10 μg collagen/mg tendon wet wt in untrained and resistance-trained men, respectively). Figure 3 compares the in vivo patellar tendon composition of untrained and chronically resistance-trained men.
Fig. 3.
In vivo tendon dry mass and collagen content in men and chronically resistance-trained (RT) men. Expression of dry mass and collagen content per tendon wet weight demonstrates functional amounts of these elements in whole tendon tissue. No differences (P > 0.05) were seen in these variables between men and RT men.
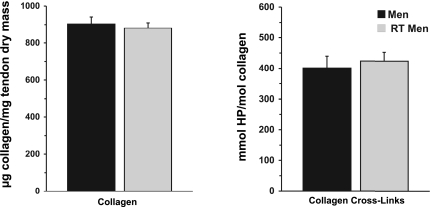
No differences (P > 0.05) were seen in collagen content of the tendon dry mass between these two groups (903 ± 38 and 881 ± 43 μg collagen/mg tendon dry mass in untrained and resistance-trained men, respectively). HP cross-linking of collagen was similarly unaltered by training (401 ± 47 and 424 ± 38 mmol HP/mol collagen in untrained and resistance-trained men, respectively, P > 0.05). Figure 4 compares the dry tendon material composition of untrained and resistance-trained men.
Fig. 4.
Dry tendon collagen content and collagen cross-linking in men and chronically resistance-trained (RT) men. Normalization of collagen to dry mass demonstrates amount of collagen in nonwater material of the tendon tissue. HP cross-links relative to collagen reveal number of cross-links per collagen in tendon tissue. Taken together, these 2 variables indicate quality of biosynthesized tendon tissue, independent of tissue water content. No differences (P > 0.05) were observed between men and RT men.
Tissue dry mass was lower in peritendon than tendon samples (361 ± 7 μg tendon dry mass/mg tendon wet wt vs. 166 ± 3 μg peritendon dry mass/mg peritendon wet wt). Collagen content per wet weight was therefore lower in peritendon than tendon tissue (320 ± 7 collagen/mg tendon wet wt vs. 123 ± 8 μg collagen/mg peritendon wet wt). Collagen content of the dry mass was only slightly higher in tendon than peritendon tissue (892 ± 20 μg collagen/mg tendon dry mass vs. 773 ± 90 μg collagen/mg peritendon dry mass). HP cross-linking was comparable in tendon and peritendon tissue (415 ± 23 and 412 ± 11 mmol HP/mol collagen in tendon and peritendon, respectively).
Coefficients of variation for triplicate injections were low for HYP (0.26 ± 0.06% and 0.83 ± 0.16% for standards and samples, respectively) and HP (0.54 ± 0.25% and 0.55 ± 0.11% for standards and samples, respectively). Coefficients of variation for assays of separate aliquots from the same tendon sample were also low (0.32% for HYP and 0.82% for HP).
DISCUSSION
The aims of this investigation were to determine the impact of sex and chronic resistance training on tissue dry mass, collagen content, and collagen cross-linking of human patellar tendon. The main findings were as follows: 1) sex decreased dry mass and tended to decrease collagen content per wet weight tendon but did not change the composition of dry tendon tissue, and 2) chronic resistance training (for 10 ± 1 yr) did not alter dry mass, collagen content, or collagen cross-linking.
As hypothesized, in vivo patellar tendon composition was altered by sex. Women had significantly less dry mass per tendon wet weight (37.6 ± 0.9% vs. 34.3 ± 0.5% dry mass) and a strong trend toward less collagen per tendon wet weight (33.9 ± 1.4% and 30.6 ± 1.1% collagen for men and women, respectively, P = 0.08). Women may synthesize less tendon material overall per tendon size, resulting in lower overall tendon dry mass and collagen content. Comparisons of tendon size and collagen synthesis in men and women support this theory. Tendon CSA relative to body size does not differ between sexes (33), but tendon collagen synthesis is significantly lower in women than men (38). Estrogen directly alters collagen kinetics (8, 15, 17), and inherently higher estrogen levels in women may therefore chronically depress collagen production, as tendon cells have estrogen receptors (8, 17). Thus, blunted collagen production via estrogen could explain the lower amount of dry mass in female tendons, as collagen comprises ∼90% of dry mass.
Women may bind more water per given amount of tendon tissue, decreasing dry mass and collagen per wet weight tendon. Estrogen and progesterone administration significantly increased plasma volume in women (49), and estrogen also increased water retention during dehydration (48). These hormones may therefore affect tendon tissue, again because of the presence of estrogen receptors on tendon cells (8, 17). Greater water infiltration of tendon tissue would increase interfibrillar spacing and decrease the overall amount of collagen in tendon tissue. The concept that increased tissue water content weakens women's tendons is supported by findings from human cadavers that air-dried plantaris tendons were 50% stronger than damp tendons (52).
Contrary to our hypotheses, collagen content per dry mass and HP cross-linking of collagen were equal in women and men. On the basis of the similarity of these structural components of tendon dry mass, collagen quality may be inferior in women. Type I collagen dominates in tendon tissue (22) and has high tensile strength and low elasticity (4, 22). Type III collagen is also present in tendon tissue; it is structurally similar to type I collagen but forms thinner, weaker fibers (4, 40). The exact proportions of type I and III collagen in healthy human tendon tissue are unknown, as are potential sex differences. It has been shown, however, that type III collagen mRNA is elevated in women compared with men (50), whereas type I collagen is decreased at tendon rupture sites (13) and type III collagen is increased in degenerated tendons (13, 45). Thus the apparent weakness of female tendons (3, 10, 21, 26) may be due to a greater amount of type III collagen in women than men.
Contrary to our hypotheses, in vivo tendon composition and the nonwater, biosynthesized material of the tendon appear unaltered by chronic resistance training in men. In light of the mechanical improvements in human tendon with exercise training (24, 25, 27, 44), the lack of change in dry mass, collagen, or cross-linking in resistance-trained men was quite surprising. Previous data from humans (24, 25, 32, 46) and animals (41, 54) demonstrate increased tendon CSA with training of sufficient duration. Increased tendon CSA with training is associated with improved force transmission and tendon strength (24, 54). Given that the members of the resistance-trained group had trained consistently for an average of 10 yr, with training programs of intensity and duration at or above those that have been shown to induce tendon alterations, it is likely that tendon CSA was larger in these resistance-trained men than in the untrained men. However, it appears that the tendon material is altered in proportion to the change in tendon size, maintaining equal proportions of dry mass, collagen content, and collagen cross-linking in trained and untrained men.
To our knowledge, this investigation provides the first report of tendon tissue dry mass, collagen content, and collagen cross-linking from in vivo healthy human patellar tendon samples. Previous data from human cadaver and animal models (5, 9, 14, 23, 47) indicate that tendon tissue is ∼30–45% dry mass. In healthy, untrained men, we show that 37.6 ± 0.9% of the tendon is composed of dry mass. Similarly, the collagen content of nonpathological human tendon has been studied only in cadavers (5, 12, 51). The present study demonstrated that collagen comprises a greater amount of the tendon's wet weight: 33.9 ± 1.4% (combined mean of all 3 groups) vs. ∼20% in previous investigations (12). Additionally, collagen was shown to comprise 90.3 ± 3.8% (combined mean of all 3 groups) of the patellar tendon dry mass vs. ∼60–75% in previous cadaver studies (5, 51). These differences are likely due to tissue sampling and collection methods (in vivo tendon biopsy vs. cadaver tissue) or differences in subject groups, as earlier data are mostly derived from older individuals (5, 51).
Perspectives and Significance
Knowledge of tissue dry mass, collagen content, and collagen cross-linking of in vivo healthy human tendon is key in understanding tendon structure and integrity, given that collagen comprises the vast majority of the nonwater tendon tissue. The present study examined the impact of two separate conditions that have been shown in the literature to alter tendon strength and collagen kinetics: sex and chronic exercise training. In the patellar tendon of women, lower overall tissue dry mass and potentially lower collagen content may indicate structural discrepancies that predispose women to tendon injury. Chronic resistance training did not alter the tendon on the level of its dry mass, collagen content, or collagen cross-linking. The lack of change in these parameters with chronic resistance training suggests that resistance training in women may not ameliorate their deficiency in tendon dry mass and collagen content. Future investigations should examine other material/molecular elements of the patellar tendon, such as other cross-link species and the proteoglycan structures, which may be altered by sex or exercise.
GRANTS
This work was supported by National Institute on Aging Grant R01 AG-20532 (to T. A. Trappe).
Acknowledgments
The authors thank the study volunteers who generously dedicated their time and efforts to the study and Bridget Sullivan for expert assistance with data collection and assay development.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl 703: 37–44, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Bank RA, TeKoppele JM, Oostingh G, Hazleman BL, Riley GP. Lysylhydroxylation and non-reducible crosslinking of human supraspinatus tendon collagen: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis 58: 35–41, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman BP, Miller SA. Equal opportunities, equal risks? Overuse injuries in female military recruits. J Public Health Med 23: 35–39, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Czarny-Ratajczak M, Latos-Bielenska A. Collagens, the basic proteins of the human body. J Appl Genet 41: 317–330, 2000. [PubMed] [Google Scholar]
- 5.Elliott DH Structure and function of mammalian tendon. Biol Rev Camb Philos Soc 40: 392–421, 1965. [DOI] [PubMed] [Google Scholar]
- 6.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem 137: 380–388, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem 53: 717–748, 1984. [DOI] [PubMed] [Google Scholar]
- 8.Frank CB, Hart DA, Shrive NG. Molecular biology and biomechanics of normal and healing ligaments—a review. Osteoarthritis Cartilage 7: 130–140, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Fullerton GD, Amurao MR. Evidence that collagen and tendon have monolayer water coverage in the native state. Cell Biol Int 30: 56–65, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Geary KG, Irvine D, Croft AM. Does military service damage females? An analysis of medical discharge data in the British armed forces. Occup Med 52: 85–90, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Gillen CM, Nishiyasu T, Langhans G, Weseman C, Mack GW, Nadel ER. Cardiovascular and renal function during exercise-induced blood volume expansion in men. J Appl Physiol 76: 2602–2610, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Gineyts E, Cloos PA, Borel O, Grimaud L, Delmas PD, Garnero P. Racemization and isomerization of type I collagen C-telopeptides in human bone and soft tissues: assessment of tissue turnover. Biochem J 345: 481–485, 2000. [PMC free article] [PubMed] [Google Scholar]
- 13.Goncalves-Neto J, Witzel SS, Teodoro WR, Carvalho-Junior AE, Fernandes TD, Yoshinari HH. Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Joint Bone Spine 69: 189–194, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res 13: 907–914, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Hansen M, Koskinen S, Petersen SG, Dossing S, Frystyk J, Flyvbjerg A, Westh E, Magnusson SP, Kjaer M, Langberg H. Ethinyl oestradiol administration in women suppresses synthesis of collagen in tendon in response to exercise. J Physiol 586: 3005–3016, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris JA, Benedict FG. A Biometric Study of Basal Metabolism in Man. Washington, DC: Carnegie Institute of Washington, 1919.
- 17.Hart DA, Archambault JM, Kydd A, Reno C, Frank CB, Herzog W. Gender and neurogenic variables in tendon biology and repetitive motion disorders. Clin Orthop Relat Res: 44–56, 1998. [PubMed]
- 18.Haskell A, Nadel ER, Stachenfeld NS, Nagashima K, Mack GW. Transcapillary escape rate of albumin in humans during exercise-induced hypervolemia. J Appl Physiol 83: 407–413, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol 103: 2068–2076, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Hutson PR, Crawford ME, Sorkness RL. Liquid chromatographic determination of hydroxyproline in tissue samples. J Chromatogr B Analyt Technol Biomed Life Sci 791: 427–430, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Jones BH, Bovee MW, Harris JM 3rd, Cowan DN. Intrinsic risk factors for exercise-related injuries among male and female army trainees. Am J Sports Med 21: 705–710, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Josza L, Kannus P. Human Tendons. Champaign, IL: Human Kinetics, 1997.
- 23.Kjaer M Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Kongsgaard M, Aagaard P, Kjaer M, Magnusson SP. Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J Appl Physiol 99: 1965–1971, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 191: 111–121, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol 88: 520–526, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol 91: 26–32, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol 534: 297–302, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol 521: 299–306, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Last JA, Reiser RM. Collagen biosynthesis. Environ Health Perspect 55: 169–177, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med 22: 675–692, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci 58: 123–127, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. Int J Exp Pathol 88: 237–240, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Sports 13: 211–223, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Magnusson SP, Kjaer M. Region-specific differences in Achilles tendon cross-sectional area in runners and non-runners. Eur J Appl Physiol 90: 549–553, 2003. [DOI] [PubMed] [Google Scholar]
- 36.McGavack TH, Kao KY. The influence of age and sex on the soluble collagen, insoluble collagen and elastin of rat tissues. Exp Med Surg 18: 104–123, 1960. [PubMed] [Google Scholar]
- 37.Michna H Morphometric analysis of loading-induced changes in collagen-fibril populations in young tendons. Cell Tissue Res 236: 465–470, 1984. [DOI] [PubMed] [Google Scholar]
- 38.Miller BF, Hansen M, Olesen JL, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol 102: 541–546, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minor RR Collagen metabolism: a comparison of diseases of collagen and diseases affecting collagen. Am J Pathol 98: 225–280, 1980. [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagaki WR, Biancalana A, Benevides GP, Gomes L. Biomechanical and biochemical properties of chicken calcaneal tendon under effect of age and nonforced active exercise. Connect Tissue Res 48: 219–228, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Narici MV, Hoppeler H, Kayser B, Landoni L, Claassen H, Gavardi C, Conti M, Cerretelli P. Human quadriceps cross-sectional area, torque and neural activation during 6 months strength training. Acta Physiol Scand 157: 175–186, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Nishiyasu T, Nagashima K, Nadel ER, Mack GW. Human cardiovascular and humoral responses to moderate muscle activation during dynamic exercise. J Appl Physiol 88: 300–307, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis 53: 359–366, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports 12: 90–98, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Shadwick RE Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol 68: 1033–1040, 1990. [DOI] [PubMed] [Google Scholar]
- 48.Stachenfeld NS, Silva C, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol 87: 1016–1025, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol 96: 1011–1018, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan BE, Carroll CC, Jemiolo B, Trappe SW, Magnusson SP, Døssing S, Kjaer M, Trappe TA. Effect of acute resistance exercise and sex on human patellar tendon structural and regulatory mRNA expression. J Appl Physiol. In press. [DOI] [PMC free article] [PubMed]
- 51.Suzuki D, Takahashi M, Abe M, Nagano A. Biochemical study of collagen and its crosslinks in the anterior cruciate ligament and the tissues used as a graft for reconstruction of the anterior cruciate ligament. Connect Tissue Res 49: 42–47, 2008. [DOI] [PubMed] [Google Scholar]
- 52.Walker LB, Harris EH, Benedict JV. Stress-strain relationship in human cadaveric plantaris tendon: a preliminary study. Med Electron Biol Eng 101: 31–38, 1964. [DOI] [PubMed] [Google Scholar]
- 53.Westh E, Kongsgaard M, Bojsen-Moller J, Aagaard P, Hansen M, Kjaer M, Magnusson SP. Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports 18: 23–30, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Woo SL, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, Garfin SR, Akeson WH. The biomechanical and biochemical properties of swine tendons—long term effects of exercise on the digital extensors. Connect Tissue Res 7: 177–183, 1980. [DOI] [PubMed] [Google Scholar]