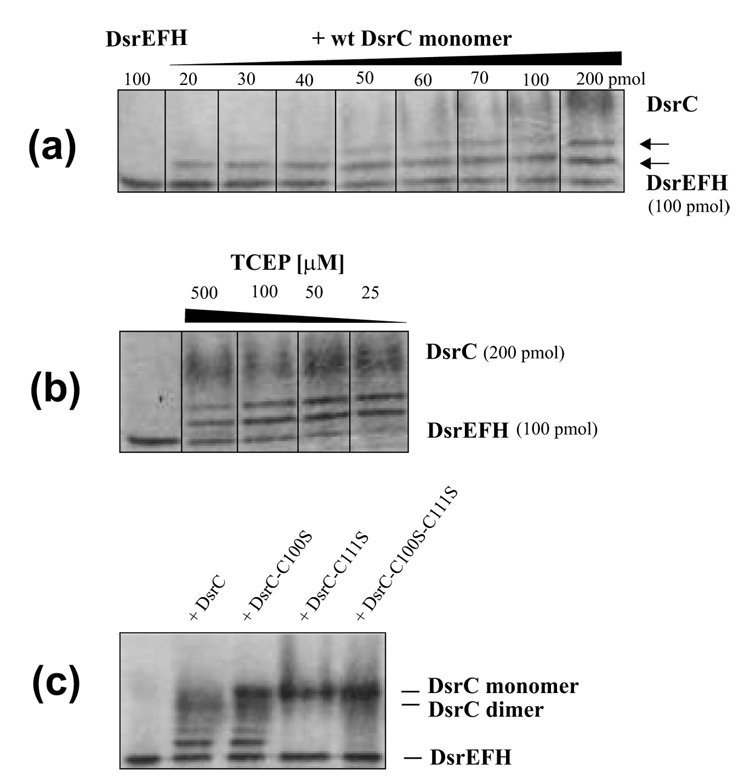
Fig. 7. The ability of DsrC to interact with DsrEFH was determined by band-shift assays.
(a) 100 pmol of DsrEFH were incubated with increasing amounts of wild type DsrC monomer in a total volume of 60 µl under the conditions specified in materials and Methods. In this series of experiments TCEP was not added. Increasing amounts of DsrC produced an additional band migrating between DsrEFH and the wt DsrC monomer. A molar ratio DsrE2F2H2 : DsrC of 2 : 1 and lower led to formation of a second distinct retarded band. Both retarded bands increased in intensity upon increase of DsrC concentration. Intensity of the original DsrEFH band decreased concomitantly. (b) Effect of reducing agent (TCEP) on formation of additional bands in band shift assays. Upon addition of 25 µM TCEP 100 pmol DsrE2F2H2 are completely retrieved in two shifted bands in the presence of 200 pmol monomeric DsrC. Higher concentrations of TCEP decrease shifted band intensities. (c) Band shift assay of DsrEFH with wild type and mutant DsrC proteins. 100 pmol DsrEFH were incubated with 200 pmol of the respective DsrC protein in the presence of 25 µM TCEP as described in Materials and Methods. Lane 1, shifted bands are clearly visible for wt DsrC and DsrC-Cys100Ser but not present in samples with DsrC-Cys111Ser and DsrC-Cys100Ser/Cys111Ser.