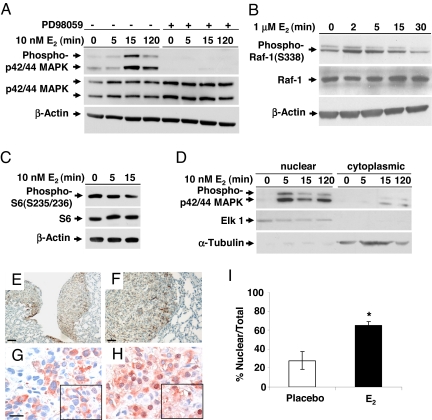
Fig. 4.
Estrogen activates p42/44 MAPK in ELT3 cells in vitro and in vivo. (A) ELT3 cells were grown in phenol red-free and serum-free media for 24 h and then stimulated with 10 nM E2 for 0, 5, 15, or 120 min. Levels of phosphorylated p42/44 MAPK and total MAPK were determined by immunoblot analysis. Pretreatment with PD98059 blocked E2-induced MAPK activation. β-Actin immunoblotting was included as a loading control. (B) Levels of phosphorylated C-Raf/Raf-1 and total Raf-1 after E2 stimulation. (C) Levels of phosphorylated S6 after E2 stimulation. (D) The nuclear and cytoplasmic fractions were separated, and levels of phospho-p42/44 MAPK were examined by immunoblot analysis. Anti-ELK1 and anti-α-tubulin were included as loading controls for the nuclear and cytosolic fractions, respectively. (E and F) Pulmonary metastases from an E2-treated mouse showed hyperphosphorylation of p42/44 MAPK. (Scale bar, 50 μM and 125 μM.) (G and H) Phospho-p42/44 MAPK (T202/Y204) immunostaining of primary tumor sections from placebo-treated (G) and E2-treated (H) mice. (Scale bar, 20 μM.) (I) Percentage of cells with nuclear immunoreactivity of phospho-p42/44 MAPK was scored from 4 random fields per section. *, P < 0.05, Student's t test.