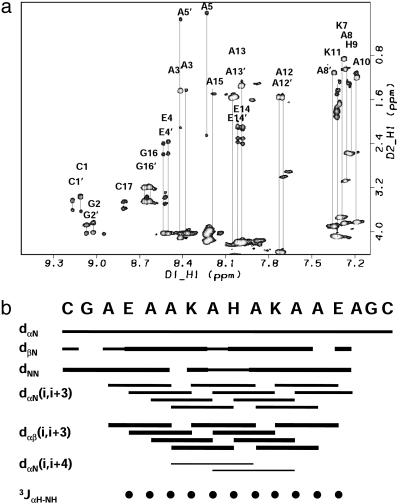
Fig. 5.
(a) Two-dimensional 1H-1H total correlation spectroscopy spectrum of the complex of [CoIII(coproporphyrinate I)]3- and Cy-AA-EK. The spectra show nice dispersion, characteristic of a structured system. (b) Summary of the sequential NOEs for the complex of [CoIII(coproporphyrinate I)]3- and Cy-AAEK. The intensities were grouped into three sets: weak, medium, and strong. The size of the bars reflects these intensities. NOEs were taken from a NOESY spectrum recorded with a mixing time of 100 ms. The three-bond 2JαNH coupling constants were taken from a high-resolution double-quantumfiltered COSY experiment and converted to φ-angle restraints by using the standard methods (36, 37). Only one of the helices is shown for clarity because the other helix gives the same pattern. The data reveal a stronger intensity for the dNN cross-peaks relative to the dαN cross-peaks, which is a sign of helicity. Another strong pattern is the dαβ(i, i + 3) cross-peaks. Although it is difficult to distinguish between 310 and α-helices from NMR data, the presence of the dαβ(i, i + 3) cross-peaks as the most intense in the spectra, the presence of dαN(i, i + 4), and the absence of dαN(i, i + 2) cross-peaks provide further support for the α-helical structure. Several unique NOEs also exist between the metalloporphyrin and the peptide and the imidazole of the histidine and other residue side chains.