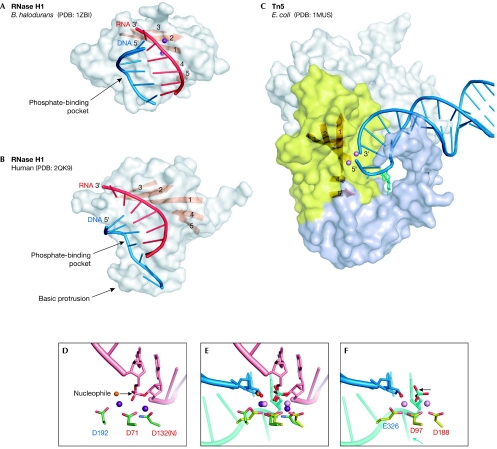
Figure 4.
Nucleic-acid complexes of Bacillus halodurans and human RNase H1 enzymes, and Escherichia coli Tn5 transposase. (A) B. halodurans RNase H1. The protein is shown in surface representation with strands of the central β-sheet shown as an orange ribbon. The portion of the nucleic acid that interacts with the protein is shown in cartoon representation with the RNA shown in red and the DNA in blue. The two metal ions at the active site are shown as purple spheres. (B) Human RNase H1. (C) Tn5 transposase in complex with a resolved DNA hairpin (blue) corresponding to the transposon end. Only one subunit of the dimer is shown. The insertion in the RNase H fold is shown in light blue. The nucleotide that is flipped out to form the DNA hairpin and is stabilized by the interactions with the insertion domain is shown in cyan stick representation. The amino-terminal domain that interacts with the nucleic acids and the carboxy-terminal dimerization domain are shown in grey. Two Mn2+ ions are shown as pink spheres. (D) Close-up view of the active site of B. halodurans RNase H1. Active-site carboxylates are shown in green and are labelled using the same colour coding as in Figs 1B and 2. The RNA strand is shown in pink with the two nucleotides joined by the scissile phosphate shown in stick representation. Two Mg2+ ions are shown as purple spheres. The attacking nucleophile is shown as an orange sphere and the direction of the attack is indicated with an arrow. (E) Superposition of the active sites of RNase H1 anf Tn5 transposase (coloured as in (D) and (F)) based on the positions of C-α atoms of the active-site carboxylates. Note that although they were not included in the superposition, the phosphate groups, metal ions and attacking nucleophiles (water in B. halodurans RNase H1 and 3′-OH in E.coli Tn5) occupy similar positions. After the non-transferred strand (cyan) is removed from the Tn5 active site, this superposition might represent the configuration of the transposon end (3′-OH group) attacking the target DNA mimicked by the RNA strand (pink) from the B. halodurans RNase H1 structure. (F) Active site of Tn5 transposase. The DNA is shown in blue (transferred strand) and cyan (non-transferred strand). The Mn2+ ions are shown as pink spheres. The 3′-OH group is indicated with a red sphere. The direction of the postulated last nucleophilic attack by a water molecule to generate this 3′-OH is indicated with an arrow. The flipped-out base is indicated with a cyan arrow. PDB, Protein Data Bank code; Tn5, transposon 5.