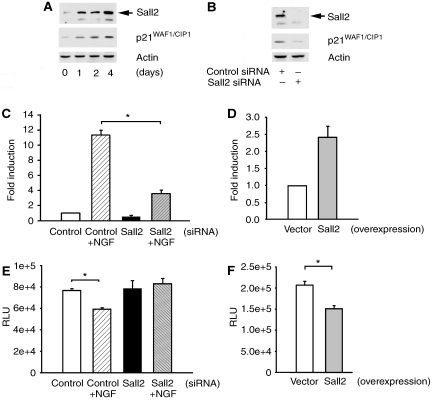
Figure 8.
Sall2 is required for NGF-dependent p21WAF1/CIP1 expression and cell cycle arrest. (A) PC12 cells were cultured in differentiation medium (DM) containing low serum for several days. Immunoblotting assayed expression of Sall2, p21WAF1/CIP1 and actin. (B) PC12 cells were transfected with control or Sall2-specific siRNA and then incubated in differentiation medium for 4 days. Immunoblotting assayed expression of Sall2, p21WAF1/CIP1 and actin. (C) Sall2 regulates p21WAF1/CIP1 promoter activity. The effect of Sall2 on the p21 promoter reporter was assayed using a firefly luciferase assay in which PC12 cells were transfected with control or Sall2-specific siRNAs (50 nM). After 48 h in the absence or presence of differentiation medium, luciferase activities were determined and normalized to β-gal. Results are the average fold induction±s.e.m. for three independent experiments performed in triplicate, *Student's t-test (P<0.005). (D) Cells were co-transfected with empty vector or HA–Sall2 together with a p21 gene reporter and β-gal and then cultured in same medium as in (C), but without NGF. After 2 days, p21 reporter activity was assayed and is presented as a bar graph as described under (C). Results are the average of fold induction calculated from three independent experiments, each performed in triplicate. (E) PC12 cells were transfected with control or Sall2-specific siRNA and then grown in the absence or presence of differentiation medium for 48 h. BrdU incorporation (in RLUs, relative luciferase units) into DNA was then assayed using a kit from Roche Inc. Results are the average±s.e.m. from three independent experiments performed in sextuplicate. *Student's t-test (P<0.001). (F) PC12 cells transfected with empty vector or HA–Sall2 were grown for 2 days in the same medium as in (E) but without NGF. BrdU incorporation was assayed and is presented as described in (D). Results are the average±s.e.m. from three independent experiments performed in sextuplicate. *Student's t-test (P<0.001).