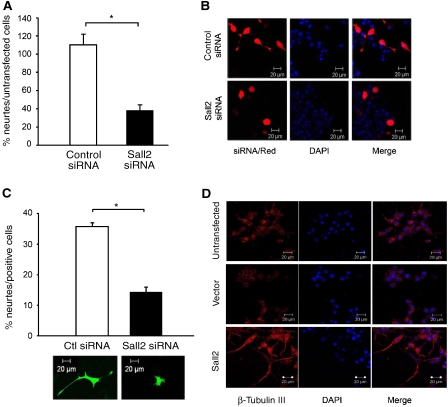
Figure 9.
Sall2 regulates neurite outgrowth from PC12 cells. (A) Equal numbers of PC12 cells transfected with control or Sall2-specific siRNA were seeded into cell culture inserts coated with collagen and then cultured in differentiation medium (DM) consisting of 1.5% serum and 100 ng/ml NGF for 2 days. Neurite outgrowth was quantified by measuring cresyl violet (A562 nm) extracted from stained neurites. Data are the mean±s.e.m. of results from three independent experiments performed in triplicate. *Student's t-test (P<0.001). The percentage of neurite outgrowth is obtained by dividing neurite formation from cells transfected with control siRNA, or Sall2 siRNA, by that from untransfected cells, and multiplying by 100. (B) PC12 cells co-transfected with control or Sall2-specific siRNA and the transfection marker dsRed were serum-starved for 24 h and then cultured with differentiation medium for 2 days. Confocal microscopy shows the phenotype of the transfected cells after 2 days in differentiation medium. (C) The bar graph shows the percentage of cells in panel B extending neurites at least one and a half cell bodies in length. About 100 cells were counted for each condition. Data are the mean±s.e.m. of results from three independent experiments, *Student's t-test (P<0.001). A representative picture of a cell transfected with control or Sall2-specific siRNA and pEGFP is shown under the bar graph. (D) PC12 cells transfected with empty vector or HA–Sall2 were cultured in the medium described under (A) but without NGF. The cells were stained with an antibody to β-tubulin III, and with DAPI, and then examined by fluorescence microscopy at × 40 magnification.