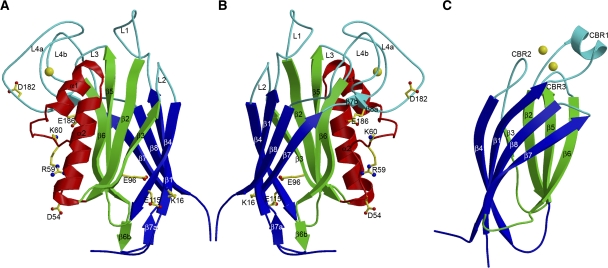
Figure 1.
Structure of the F-spondin domain of mindin and comparison with a Ca2+-dependent C2 domain. (A) Side view of mindin-FS (ribbon diagram). The two four-stranded β-sheets, β6β5β2β3 (front) and β4β1β8β7 (back), are green and blue, respectively. Helices α1 and α2 are red. The bound Ca2+ ion is yellow. Loops L1–L4 at the top of the molecule are cyan. Residues mutated to alanine to localize the integrin-binding site are drawn in ball-and-stick representation, with carbon atoms in yellow, oxygen atoms in red and nitrogen atoms in blue. (B) Mindin-FS is rotated ∼180° about the vertical axis with respect to the view in (A). (C) Side view of the C2 domain of cytosolic phospholipase A2 (PDB accession code 1RLW). The orientation is similar to that of mindin-FS in (B). The two four-stranded β-sheets, β4β1β8β7 (front) and β6β5β2β3 (back), are blue and green, respectively. The bound Ca2+ ion is yellow. Calcium-binding loops CBR1–CBR3 are cyan.