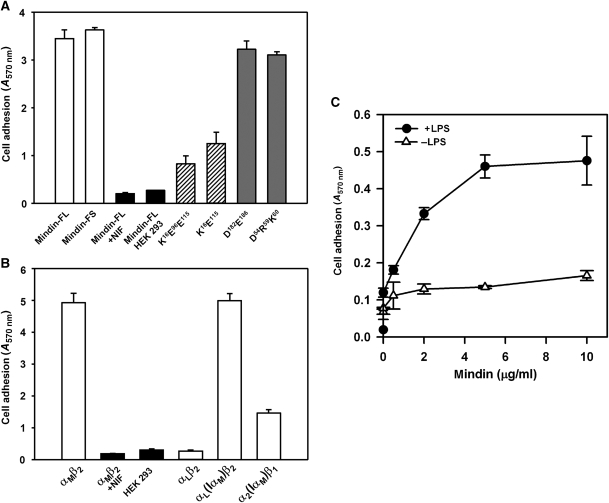
Figure 4.
Cell adhesion to mindin. (A) αMβ2-mediated cell adhesion to mindin. A total of 2 × 106 αMβ2-expressing or mock-transfected HEK 293 cells were allowed to adhere to 24-well non-tissue culture polystyrene plates precoated with recombinant mindin-FL, mindin-FS or its different mutants. After incubation at 37°C for 30 min, unbound cells were removed by three washes with DPBS and adherent cells were quantified by staining with crystal violet, measuring absorption at 570 nm. Specificity of cell adhesion was verified using the αMβ2-specific antagonist NIF, mock-transfected cells and biotinylated IgG or ovalbumin as the substrate (not shown). Data shown are the means±s.d. of triplicate wells and are representative of three independent experiments. (B) The αMβ2-mediated cell adhesion to mindin is mediated by its αM I domain. Cell adhesion to mindin-FL by different integrin receptors, including αMβ2, αLβ2, αL(IαM)β2 and α2(IαM)β1, where the αL or α2 I domain was replaced with that of αM, were determined as above. Specificity was verified by the addition of the αMβ2-specific antagonist NIF and by using mock-transfected HEK 293 cells. Data shown are the means ±s.d. of triplicate wells and are representative of two independent experiments. (C) Neutrophil adhesion to mindin is enhanced by LPS. Murine neutrophils were added to mindin-coated 24-well plates (0.5 × 106 cells per well) in the presence or absence of 1 μg/ml LPS. After 10 min at 37°C, unbound cells were removed by washing with PBS and bound cells were quantified by crystal violet staining, measuring absorption at 570 nm.