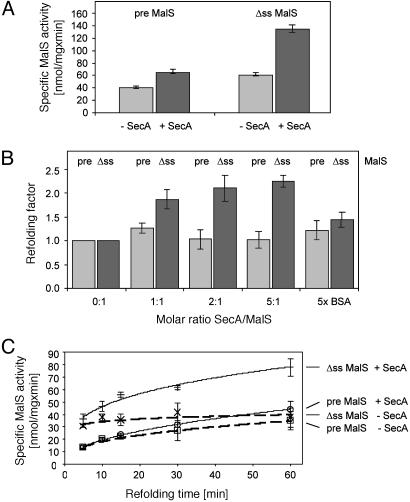
Fig. 3.
Refolding of MalS. (A) Refolding of pre- and ss-MalS in the presence or absence of SecA. Chemically denatured MalS was diluted 1:50 in 100 mM Tris, pH 7.5. SecA was added in a 2-fold molar excess (black) or an equivalent of SecA buffer (gray) for 1 h. After refolding, 2 mM MalS substrate p-nitrophenylhexaoside was added to determine MalS activity. (B) Refolding of MalS in the presence of various concentrations of SecA. Refolding was done as described in A except that SecA/MalS ratios varied as indicated and 5-fold molar excess of BSA was used as a negative control. The added SecA volume was held constant to exclude possible salt effects of the SecA buffer. The refolding factors given represent the enzymatic activity of MalS after refolding in the presence of SecA divided by the enzymatic activity after refolding without SecA. Results of samples containing ss-MalS (Δss) are shown in black and samples containing pre-MalS (pre), in gray. (C) Time-dependent refolding of MalS in the presence or absence of SecA. Refolding was done as described in A except that MalS substrate was added after various times before determining MalS activity. Bars indicate standard deviation.