Abstract
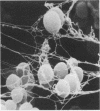
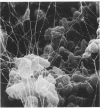
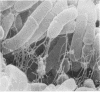
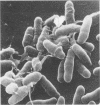
Corynebacterium spp. are responsible for an important minority of cases of colonization of cerebrospinal fluid shunts used for the treatment of hydrocephalus. In common with coagulase-negative staphylococci, they present a serious therapeutic problem because they are often resistant to multiple antibiotics. We studied the morphologies of coryneforms in colonized hydrocephalus shunts removed from patients and observed extracellular slime similar in appearance to that seen in coagulase-negative staphylococci. We also studied a series of such isolates from other cases of hydrocephalus shunt colonization using an established laboratory model and consistently observed slime production in these shunts as well. We propose that this might be a further reason for failure to eradicate these organisms without shunt removal as well as a factor in their pathogenesis in device-related infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen K. D., Green H. T. Infections due to a 'Group JK' corynebacterium. J Infect. 1986 Jul;13(1):41–44. doi: 10.1016/s0163-4453(86)92256-5. [DOI] [PubMed] [Google Scholar]
- Altwegg M., Záruba K., von Graevenitz A. Corynebacterium group JK peritonitis in patients on continuous ambulatory peritoneal dialysis. Klin Wochenschr. 1984 Aug 16;62(16):793–794. doi: 10.1007/BF01721780. [DOI] [PubMed] [Google Scholar]
- Athalye M., Noble W. C., Mallet A. I., Minnikin D. E. Gas chromatography-mass spectrometry of mycolic acids as a tool in the identification of medically important coryneform bacteria. J Gen Microbiol. 1984 Mar;130(3):513–519. doi: 10.1099/00221287-130-3-513. [DOI] [PubMed] [Google Scholar]
- Bayston R., Barsham S. Catheter colonisation: a laboratory model suitable for aetiological, therapeutic and preventive studies. Med Lab Sci. 1988 Jul;45(3):235–239. [PubMed] [Google Scholar]
- Bayston R., Hart C. A., Barnicoat M. Intraventricular vancomycin in the treatment of ventriculitis associated with cerebrospinal fluid shunting and drainage. J Neurol Neurosurg Psychiatry. 1987 Nov;50(11):1419–1423. doi: 10.1136/jnnp.50.11.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayston R., Higgins J. Biochemical and cultural characteristics of "JK" coryneforms. J Clin Pathol. 1986 Jun;39(6):654–660. doi: 10.1136/jcp.39.6.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayston R., Leung T. S., Wilkins B. M., Hodges B. Bacteriological examination of removed cerebrospinal fluid shunts. J Clin Pathol. 1983 Sep;36(9):987–990. doi: 10.1136/jcp.36.9.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayston R., Penny S. R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- Bolton W. K., Sande M. A., Normansell D. E., Sturgill B. C., Westervelt F. B., Jr Ventriculojugular shunt nephritis with Corynebacterium bovis. Successful therapy with antibiotics. Am J Med. 1975 Sep;59(3):417–423. doi: 10.1016/0002-9343(75)90401-5. [DOI] [PubMed] [Google Scholar]
- Gavin S. E., Leonard R. B., Briselden A. M., Coyle M. B. Evaluation of the rapid CORYNE identification system for Corynebacterium species and other coryneforms. J Clin Microbiol. 1992 Jul;30(7):1692–1695. doi: 10.1128/jcm.30.7.1692-1695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombert M. E., Landesman S. H., Corrado M. L., Stein S. C., Melvin E. T., Cummings M. Vancomycin and rifampin therapy for Staphylococcus epidermidis meningitis associated with CSF shunts: report of three cases. J Neurosurg. 1981 Oct;55(4):633–636. doi: 10.3171/jns.1981.55.4.0633. [DOI] [PubMed] [Google Scholar]
- Hande K. R., Witebsky F. G., Brown M. S., Schulman C. B., Anderson S. E., Jr, Levine A. S., MacLowery J. D., Chabner B. A. Sepsis with a new species of Corynebacterium. Ann Intern Med. 1976 Oct;85(4):423–426. doi: 10.7326/0003-4819-85-4-423. [DOI] [PubMed] [Google Scholar]
- Holland S. P., Pulido J. S., Miller D., Ellis B., Alfonso E., Scott M., Costerton J. W. Biofilm and scleral buckle-associated infections. A mechanism for persistence. Ophthalmology. 1991 Jun;98(6):933–938. doi: 10.1016/s0161-6420(91)32199-7. [DOI] [PubMed] [Google Scholar]
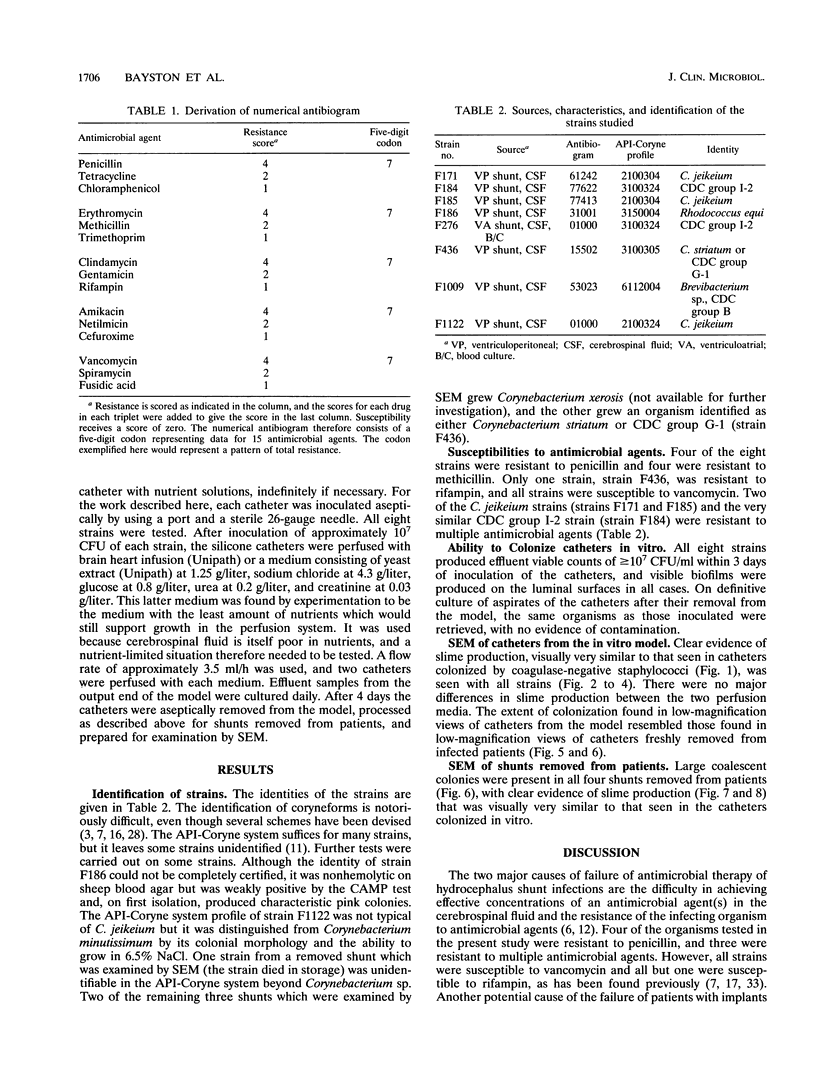
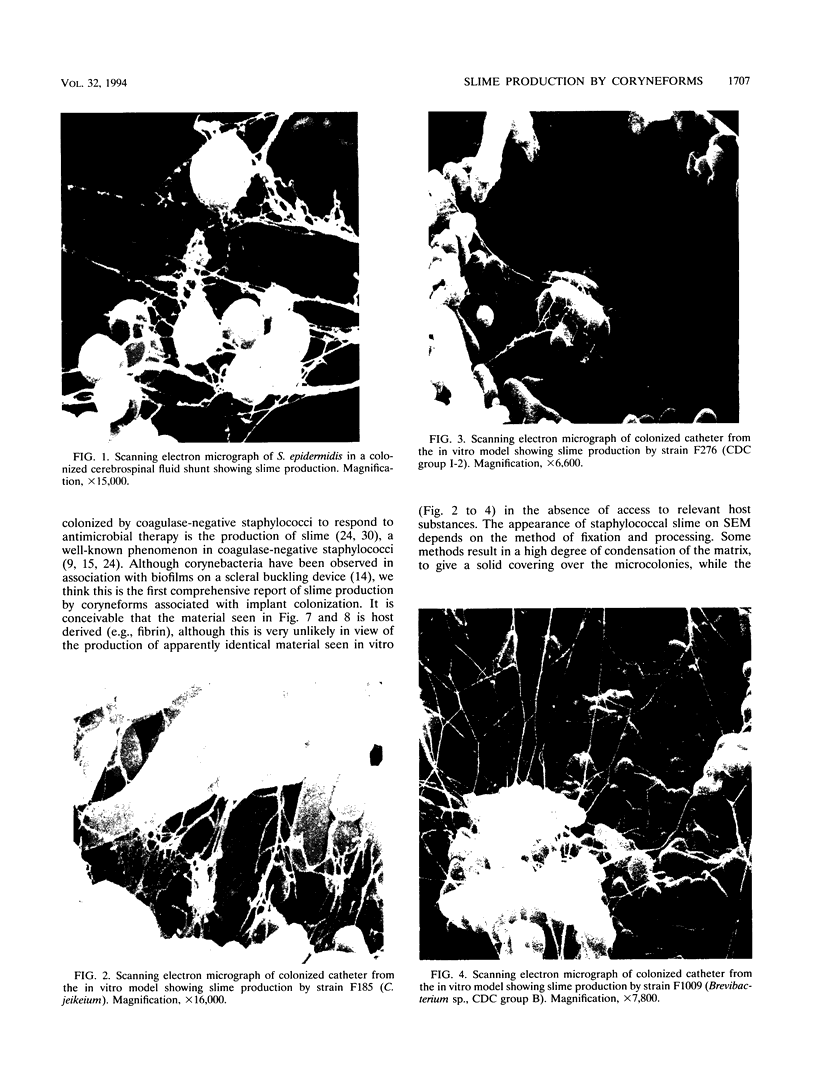
- Hussain M., Wilcox M. H., White P. J. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol Rev. 1993 Apr;10(3-4):191–207. doi: 10.1111/j.1574-6968.1993.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Jackman P. J. Classification of Corynebacterium species from axillary skin by numerical analysis of electrophoretic protein patterns. J Med Microbiol. 1982 Nov;15(4):485–492. doi: 10.1099/00222615-15-4-485. [DOI] [PubMed] [Google Scholar]
- Jadeja L., Fainstein V., LeBlanc B., Bodey G. P. Comparative in vitro activities of teichomycin and other antibiotics against JK diphtheroids. Antimicrob Agents Chemother. 1983 Aug;24(2):145–146. doi: 10.1128/aac.24.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K., Weinstein L. Diphtheroid infections of man. Ann Intern Med. 1969 May;70(5):919–929. doi: 10.7326/0003-4819-70-5-919. [DOI] [PubMed] [Google Scholar]
- Keren G., Geva T., Bogokovsky B., Rubinstein E. Corynebacterium Group JK pathogen in cerebrospinal fluid shunt infection. Report of two cases. J Neurosurg. 1988 Apr;68(4):648–650. doi: 10.3171/jns.1988.68.4.0648. [DOI] [PubMed] [Google Scholar]
- Leung A. C., Orange G., Henderson I. S., Kennedy A. C. Diphtheroid peritonitis associated with continuous ambulatory peritoneal dialysis. Clin Nephrol. 1984 Oct;22(4):200–205. [PubMed] [Google Scholar]
- Moss S. W., Gary N. E., Eisinger R. P. Nephritis associated with a diphtheroid-infected cerebrospinal fluid shunt. Am J Med. 1977 Aug;63(2):318–319. doi: 10.1016/0002-9343(77)90248-0. [DOI] [PubMed] [Google Scholar]
- O'Regan S., Makker S. P. Shunt nephritis: demonstration of diphtheroid antigen in glomeruli. Am J Med Sci. 1979 Sep-Oct;278(2):161–165. [PubMed] [Google Scholar]
- Peters G., Pulverer G. Pathogenesis and management of Staphylococcus epidermidis 'plastic' foreign body infections. J Antimicrob Chemother. 1984 Dec;14 (Suppl 500):67–71. doi: 10.1093/jac/14.suppl_d.67. [DOI] [PubMed] [Google Scholar]
- Pierard D., Lauwers S., Mouton M. C., Sennesael J., Verbeelen D. Group JK corynebacterium peritonitis in a patient undergoing continuous ambulatory peritoneal dialysis. J Clin Microbiol. 1983 Oct;18(4):1011–1014. doi: 10.1128/jcm.18.4.1011-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekate H. L., Ruch T., Nulsen F. E. Diphtheroid infections of cerebrospinal fluid shunts. The changing pattern of shunt infection in Cleveland. J Neurosurg. 1980 Apr;52(4):553–556. doi: 10.3171/jns.1980.52.4.0553. [DOI] [PubMed] [Google Scholar]
- Riebel W., Frantz N., Adelstein D., Spagnuolo P. J. Corynebacterium JK: a cause of nosocomial device-related infection. Rev Infect Dis. 1986 Jan-Feb;8(1):42–49. doi: 10.1093/clinids/8.1.42. [DOI] [PubMed] [Google Scholar]
- Riley P. S., Hollis D. G., Utter G. B., Weaver R. E., Baker C. N. Characterization and identification of 95 diphtheroid (group JK) cultures isolated from clinical specimens. J Clin Microbiol. 1979 Mar;9(3):418–424. doi: 10.1128/jcm.9.3.418-424.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozdzinski E., Kern W., Schmeiser T., Kurrle E. Corynebacterium jeikeium bacteremia at a tertiary care center. Infection. 1991 Jul-Aug;19(4):201–204. doi: 10.1007/BF01644945. [DOI] [PubMed] [Google Scholar]
- Sheth N. K., Franson T. R., Sohnle P. G. Influence of bacterial adherence to intravascular catheters on in-vitro antibiotic susceptibility. Lancet. 1985 Dec 7;2(8467):1266–1268. doi: 10.1016/s0140-6736(85)91552-1. [DOI] [PubMed] [Google Scholar]
- Stamm W. E., Tompkins L. S., Wagner K. F., Counts G. W., Thomas E. D., Meyers J. D. Infection due to Corynebacterium species in marrow transplant patients. Ann Intern Med. 1979 Aug;91(2):167–173. doi: 10.7326/0003-4819-91-2-167. [DOI] [PubMed] [Google Scholar]
- Traub W. H. Multiple drug-resistant Corynebacteriaceae: in vitro and in vivo (murine) studies. Chemotherapy. 1985;31(5):372–382. doi: 10.1159/000238362. [DOI] [PubMed] [Google Scholar]