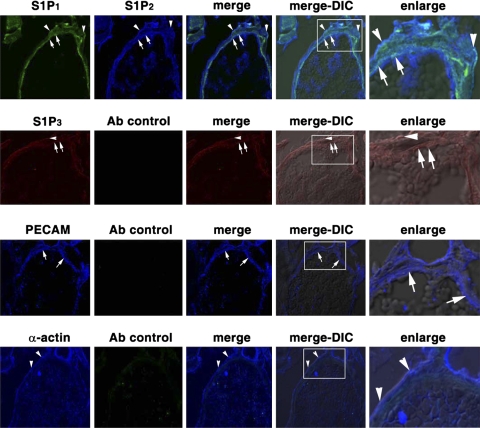
Fig. 3.
Expression of S1P1 and S1P2 in the venule of the cremaster muscle circulatory bed. Cremaster specimens were surgically removed from SD rats. After sections were fixed with 4% paraformaldehyde and embedded in OCT compound, frozen 8-μm-thick sections were immunostained with primary antibodies and then with Alexa 488 (green)-, Alexa 594 (red)-, or Alexa 647 (blue)-conjugated secondary antibodies and analyzed by a confocal microscope. Top to bottom: goat anti-S1P1 and rabbit anti-S1P2 primary antibodies and Alexa 488-donkey anti-goat and Alexa 647-donkey anti-rabbit secondary antibodies; specimens analyzed with Alexa 488 and Alexa 647 filters (row 1); goat anti-S1P3 primary antibody and Alexa 594-donkey anti-goat secondary antibody; specimens analyzed with Alexa 594 and Alexa 647 filters (row 2); mouse anti-rat platelet endothelial cell adhesion molecule (PECAM) primary antibody and Alexa 647-donkey anti-mouse and Alexa 488-donkey anti-goat secondary antibodies; specimens analyzed with Alexa 488 and Alexa 647 filters (row 3); mouse anti-α-actin primary antibody and Alexa 647-donkey anti-mouse secondary antibody; specimens analyzed with Alexa 488 and Alexa 647 filters (row 4). Endothelium (arrows) and smooth muscle cells (arrowheads) were identified by staining with PECAM (row 3) and α-actin (row 4) antibodies, respectively. S1P1 and S1P2 receptors are expressed in endothelium (arrows) and smooth muscle cells (arrowheads) of cremaster muscle venules (row 1), and S1P3 receptors were weakly detected in the venules of cremaster muscle vasculature (row 2). Enlarge, enlarged images of boxed areas in merge-differential interference contrast (DIC) image; merge-DIC, fluorescence merged images overlapped with DIC images.