Abstract
Toll-like receptors (TLRs) represent the first line of host defense against microbial infection and play a pivotal role in both innate and adaptive immunity. TLRs recognize invading pathogens through molecular pattern recognition, transduce signals via distinct intracellular pathways involving a unique set of adaptor proteins and kinases, and ultimately lead to the activation of transcription factors and inflammatory responses. Among 10 TLRs identified in humans, at least two exist in the heart, i.e., TLR2 and TLR4. In addition to the critical role of these in mediating cardiac dysfunction in septic conditions, emerging evidence suggests that the TLRs can also recognize endogenous ligands and may play an important role in modulating cardiomyocyte survival and in ischemic myocardial injury. In animal models of ischemia-reperfusion injury or in hypoxic cardiomyocytes in vitro, the administration of a sublethal dose of lipopolysaccharide, which signals through TLR4, reduces subsequent myocardial infarction, improves cardiac functions, and attenuates cardiomyocyte apoptosis. By contrast, a systemic deficiency of TLR2, TLR4, or myeloid differentiation primary-response gene 88, an adaptor critical for all TLR signaling, except TLR3, leads to an attenuated myocardial inflammation, a smaller infarction size, a better preserved ventricular function, and a reduced ventricular remodeling after ischemic injury. These loss-of-function studies suggest that both TLRs contribute to myocardial inflammation and ischemic injury in the heart although the exact contribution of cardiac (vs. circulatory cell) TLRs remains to be defined. These recent studies demonstrate an emerging role for TLRs as a critical modulator in both cell survival and tissue injury in the heart.
Keywords: apoptosis, cardiomyocytes, inflammation, innate immune, interleukin-1 receptor-associated kinase, ischemia-reperfusion, myeloid differentiation primary-response gene 88 adapter-like protein, myocardial infarction, nuclear factor-κB, remodeling
innate immune systems such as those mediated via Toll-like receptors (TLRs) represent the first line of defense against invading microbial pathogens. These receptors play a critical role in both innate and adaptive immunity (1, 102, 141). There are at least 10 TLRs identified so far in humans, and they recognize and specifically bind to a variety of pathogenic agonists such as lipopeptide (via TLR2), double-stranded RNA (via TLR3), lipopolysaccharide (LPS) (via TLR4), flagellin (via TLR5), and deoxycytidylate-phosphate-deoxyguanylate DNA (via TLR9) by molecular pattern recognition (1, 73). The stimulation of these TLRs leads to, through their specific intracellular signaling pathways, the activation of various downstream transcription factors and the ultimate production of inflammatory cytokines in host immune cells. In addition to their pivotal role in host immune defense against invading pathogens, TLRs, demonstrated by emerging evidence from the past 5–10 years, appear capable of responding to stress and modulating inflammation and tissue damage following noninfectious insults such as hypoxia and ischemia in various tissues (107), such as the lung (68), liver (121), brain (84, 144), and heart (28, 36, 118, 136, 149).
In mice, the heart expresses at least six receptors involved in TLR signaling, namely TLR2, TLR3, TLR4, TLR5, TLR7, and TLR9 (17). A pathogenic ligand stimulation of TLR2, TLR4, TLR5, and TLR9 can lead to the activation of the NF-κB pathway and cardiomyocyte contractile dysfunction (17, 81, 169). The two most-studied TLRs in the heart are TLR2 and TLR4 (41, 79, 80, 109, 169). Animal studies have indicated that these receptors are in part responsible for cardiac dysfunction in certain pathological conditions characterized in either gram-negative or gram-positive bacterial infection, such as endotoxemia (79, 109), peptidoglycan-associated lipoprotein (169), and staphylococcus aureus (80). In the absence of a pathogen, an insult induced by transient tissue hypoxia and ischemia can induce a dramatic innate immune response in the myocardium, which has an adverse impact on cardiac anatomy and function. Probably for this very reason, the role of TLR signaling in ischemic myocardial injury (and in other cardiovascular diseases) has been intensely studied in both human and animals (39). However, population-based studies designed to determine the impact of TLR4 polymorphism on the risk of myocardial infarction (MI) are so far inconclusive, and the data seem conflicting. Some studies suggest that individuals with the single nucleotide polymorphism of TLR4 Asp299Gly, who have an impaired host immune response toward LPS stimulation, have a lower risk of MI (9, 15, 54), whereas others suggest an increased (32) or the same level of risk (83) of MI in the polymorphism compared with the control population. In animal models of ischemic cardiac injury, the role of TLRs is incompletely defined. For example, the systemic administration of a sublethal dose of LPS, which signals through TLR4, reduced the subsequent ischemic MI and improved cardiac functions both in vivo and in isolated hearts (13, 18, 89, 99, 106, 138, 155, 166, 167). The activation of TLR4-myeloid differentiation primary-response gene 88 (MyD88) signaling also protects cardiomyocytes against apoptosis (26, 170). By contrast, in the absence of systemic TLR4 stimulation, mice deficient for TLR4 exhibited a reduced myocardial inflammation and infarction compared with wild-type (WT) mice in an in vivo model of ischemia-reperfusion (I/R) injury, suggesting that TLR4 mediates ischemic injury in the heart (28, 118). These studies demonstrate an emerging role for TLRs as a critical modulator in both cell survival and tissue injury in the heart. This article reviews the experimental evidence that demonstrates 1) the role of TLR signaling in modulating cardiomyocyte apoptosis, 2) LPS preconditioning against ischemic myocardial injury, and 3) the emerging role of TLR signaling in ischemic myocardial injury as well as inflammation as demonstrated by the loss-of-function studies in mice.
Discovery of Toll and TLR
Toll, meaning “amazing” and “wonderful” in German, was first described by Christiane Nüsslein-Volhard and colleagues in 1985 when they were studying the genetic mechanisms that control early embryonic development in the fruit fly Drosophila (4, 5). They discovered that Drosophila females that lacked Toll gene activity produced dorsalized embryos, in which all embryonic cells behaved like the dorsal cells of the WT embryo. As a result, the Toll-deficient embryos lacked any dorsal-ventral polarity. Together with Edward B. Lewis and Eric Wieschaus, Nüsslein-Volhard won the Nobel Prize in Physiology or Medicine in 1995 for their discoveries concerning the genetic control of early embryonic development (http://nobelprize.org/nobel_prizes/medicine/laureates/1995/). Ten years after Toll was discovered, investigators found that Toll also played an important role in Drosophila host immunity against bacteria and fungi (85). A fly deficient of Toll died quickly of bacterial or fungus infection. In 1997, a group of investigators led by P. Medzhitov and C. A. Janeway, Jr., discovered that a human homolog of the Drosophila Toll protein was a transmembrane protein with an extracellular domain consisting of a leucine-rich repeat domain and cytoplasmic domain homologous to the cytoplasmic domain of human interleukin-1 (IL-1) receptor (103). The expression of a constitutively active mutant of human Toll or Toll-like protein in the cell line activated the NF-κB pathway and the expression of NF-κB-mediated proinflammatory cytokines IL-1, IL-6, and IL-8. Between 1998 and 1999, two groups of investigators independently found that mice with mutated Lps gene, either a missense point (Pro→His, C3H/HeJ strain) or null mutation (C57BL/10 ScCr strain), conferred a natural hyporesponsiveness to endotoxin (120, 123). Through targeted gene (Lps) disruption, Hoshino and colleagues (55) generated TLR4-deficient mice that were resistant to LPS. These pioneering studies firmly established TLR4 as the receptor for LPS, a wall component of gram-negative bacteria.
TLR-Signaling Pathways
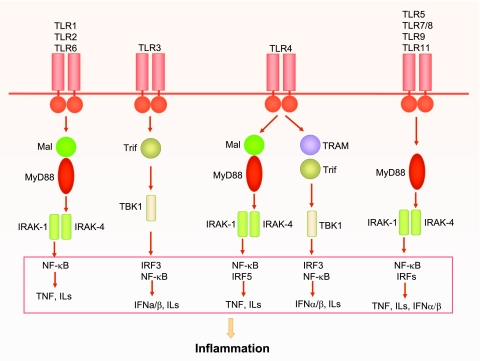
There are 10 TLRs identified in humans (12, 101). Upon binding to its specific ligand via pattern recognition, TLRs recruit and activate various downstream kinases such as IL-1 receptor-associated kinase (IRAK)-1, IRAK-4, and TNF receptor-associated factor (TRAF)-family member-associated NF-κB activator-binding kinase 1 (TBK1) via a specific set of adaptors (Fig. 1). There are five Toll/IL-1 receptor (TIR) domain-containing adaptors, namely MyD88, MyD88 adaptor-like protein (Mal), TIR-domain-containing adaptor protein inducing IFN-β-mediated transcription factor (TRIF), TRIF-related adaptor molecule (TRAM), and a sterile α- and armadillo-motif-containing protein (SARM) (112). TLRs interact with their respective adaptor(s) via the homologous binding of their unique TIR domains present in both the receptors and the adaptor molecules. Based on the specific adaptors recruited to TLRs, TLR signaling can be divided to two general pathways, namely, MyD88- and Trif-dependent (or MyD88-independent) pathways. The two distinct signaling pathways lead to the production of proinflammatory cytokines and type 1 IFN, respectively (112). As illustrated in Fig. 1, all TLRs, with the exception of TLR3, signal through MyD88-dependent pathways. In TLR2 (and TLR1 and TLR6) and TLR4 signaling, Mal is required for recruiting MyD88 to the receptors (37). By contrast, in TLR3 and TLR4 signaling, a MyD88-independent pathway is initiated and another adaptor, Trif, is proven critical (53, 162).
Fig. 1.
Toll-like receptor (TLR) signaling. All TLRs are transmembrane proteins with a large extra-cellular domain containing leucine-rich repeats and a unique cytoplasmic Toll/IL-1 receptor (TIR) domain. TLRs exist in dimmers, and all TLR family members, except TLR3, signal through the key adaptor myeloid differentiation primary-response gene 88 (MyD88) to recruit downstream interleukin (IL)-1 receptor-associated kinases (IRAKs). In some TLR signaling, such as TLR2 and TLR4, MyD88 adaptor-like protein (Mal) is required for recruiting MyD88 to their receptors, whereas in others, such as TLR5, TLR7, TLR9, and TLR11, Mal is not required. TLR1 and TLR2 or TLR2 and TLR6 form heterodimers that signal through Mal/MyD88. TLR3 signals through the adaptor TIR-domain-containing adaptor protein inducing interferon-β (IFN-β)-mediated transcription-factor (Trif), which recruits and activates TNF receptor-associated factor-family member-associated NF-κB activator-binding kinase 1 (TBK1). In addition to Mal/MyD88-dependent pathway, TLR4 can also signal through a MyD88-independent pathway that activates TBK1 via Trif-related adaptor molecule (TRAM)-Trif-dependent mechanism. TLR5, TLR7/8, TLR9, and TLR11 use only MyD88 as its signaling adaptor. These kinases ultimately activate transcription factors such as nuclear factor-κB (NF-κB) and IFN regulatory factor (IRFs), which result in production of various proinflammatory cytokines such as tumor necrosis factor (TNF), ILs, and IFNs.
The first and best known TLR is TLR4 (55, 120, 123). For LPS recognition, three additional proteins are required, including LPS binding protein (161), CD14 (160), and MD-2 (135). TLR4 signals via the two distinct pathways, MyD88-dependent and Trif-dependent pathways (112). In MyD88-dependent pathway, activated TLR4 recruits downstream IRAKs through the adapter proteins Mal and MyD88. Following a cascade of kinase activation as described below, this pathway ultimately leads to the activation of NF-κB and production of proinflammatory cytokine such as TNF and ILs (Fig. 1). In Trif-dependent pathway, TLR4 signals through TRAM-Trif that results in TBK1 activation and a downstream stimulation of IRF3 and production of IFN (Fig. 1). In TLR2 signaling, TLR2 dimerizes with either TLR1 or TLR6. The heterodimers recruit and activate IRAK4/1 via a Mal/MyD88-dependent mechanism and ultimately lead to the induction of cytokines. TLR2 signaling does not induce a production of IFN (Fig. 1).
As stated above, MyD88 is one of the five TIR domain-containing adaptors in TLR signaling (112) and plays a critical role in the signaling of all TLRs except TLR3 (64, 104, 108) (Fig. 1). It was originally isolated as one of the 12 myeloid differentiation primary response genes (91). It has a NH2-terminal death domain (DD) and a COOH-terminal TIR domain. MyD88 binds to the TLR complex via the TIR-TIR domains and in turn recruits the downstream kinase IRAKs via their DD-DD interaction. Like TLR4−/− mice (55), MyD88−/− mice (72) lack the ability to respond to LPS although MyD88-independent mechanisms in TLR4 signaling exist (53, 74, 162) (Fig. 1).
First described as a signal transducer for the proinflammatory cytokine IL-1 (20), IRAK-1 was later implicated in the signal transduction of other members of the TLR/IL-1R family. Four different IRAK-like molecules have been identified: two active kinases, IRAK-1 and IRAK-4, and two inactive kinases, IRAK-2 and IRAK-like molecule (IRAK-M) (63). All IRAKs are multidomain proteins, consisting of a conserved NH2-terminal DD and a central kinase domain (63). The DD is a protein interaction motif implicated in the binding of IRAKs to the upstream adaptor protein MyD88. The recruited IRAK-1 becomes phosphorylated by activated IRAK-4. Phosphorylated IRAK-1 binds to TRAF-6 (21, 158). The IRAK-1-TRAF-6 complex then activates transforming growth factor-β-activated protein kinase 1 (TAK1) through a process involving the cytosol translocation of TAK1- and two TAK1-binding proteins from membrane to cytosol and the ubiquitination of TRAF-6 (69, 140). Activated TAK1 then phosphorylates IKK-α/β as well as JNK/p38 kinases, leading to the activation of NF-κB and activator protein-1, respectively (153).
It is noteworthy that several other signaling molecules (not shown in Fig. 1) are involved in the feedback regulation of TLR signaling. These include IRAK-M (82), MyD88s (19, 65), an alternative splice product of MyD88, and SARM (22). These negative regulators may play an important role in LPS tolerance, a transient state of LPS refractoriness after the initial, sublethal exposure to LPS. Several mechanisms, among others, have been proposed to be responsible for endotoxin tolerance, including 1) the downregulation of the surface expression of TLR4 (111, 132); 2) the inhibition of TLR signaling by IRAK-M, which prevents the dissociation of IRAK-1/4 from MyD88 and the formation of IRAK-TRAF-6 complexes (82) and by MyD88s, which block IRAK-4 binding to MyD88, as the negative regulators of TLR signaling (19); and 3) SARM, an adaptor protein that specifically blocks TRIF-dependent and MyD88-independent NF-κB activation and gene induction (22).
TLR Signaling Modulates Cardiomyocyte Apoptosis
Functional relevance of apoptosis in cardiac diseases.
Although ischemia with associated hypoxia is the main initial event leading to ischemic MI, the molecular mechanisms involved in the consequent cardiomyocyte death are not completely defined and have been studied extensively. Several lines of evidence suggest that apoptosis, or programmed cell death, and the caspase proteases central to the apoptotic process could play a role in the pathogenesis of cardiac disease including ischemic myocardial injury. Cardiomyocyte apoptosis has been found in the injured myocardium of patients who had died of MI (62, 116, 131) and in animal models of infarction (43, 71, 130). Cardiomyocyte apoptosis is particularly prominent in reperfusion injury (43) and mainly localized in the border zone of the histological infarction area and a few in remote noninfarcted myocardiums (131). In animal models, the transgenic cardiac expression and conditional activation of procaspase-8 led to a significant cardiac apoptosis and a lethal-dilated cardiomyopathy; both can be prevented by an administration of a broad-spectrum caspase inhibitor (157). In a mouse model of chronic pressure overload, the conditional deletion of cardiac gp-130 cytokine receptor led to the rapid onset of dilated cardiomyopathy and a massive induction of cardiomyocyte apoptosis versus that in the control mice which exhibit compensatory hypertrophy, thus suggesting that cardiomyocyte apoptosis is a critical element in the transition between compensatory cardiac hypertrophy and heart failure (52). In a rat model of I/R injury, an intravenous administration of benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone, a broad caspase inhibitor, resulted in a modest but significant reduction in infarct size (33.4%) and cardiomyocyte apoptosis (72.1%) (163), demonstrating a link between cardiomyocyte apoptosis and ischemic myocardial injury. In a similar model of I/R injury, the adenovirus-mediated gene expression of a constitutive active Akt (PKB) or human insulin-like growth factor I was sufficient to attenuate cardiomyocyte apoptosis and reduce infarct size (23, 42, 97, 98). Moreover, the transgene expression of the dominant-negative (dn) mutant of Fas-associated DD protein (FADD), an adaptor protein critical for the death receptor (DR) pathway of apoptosis, inhibited apoptosis of hypoxic cardiomyocytes and reduced MI after I/R injury (24, 25). Thus the modulation of at least some targets in the apoptotic signaling pathways appears capable of mediating a meaningful anatomic and functional rescue following pressure overload or I/R injury.
Signaling pathways of cardiomyocyte apoptosis.
The execution of apoptosis is a tightly regulated and energy-dependent process that requires specialized cellular machinery (7, 8, 45). The central component of this machinery is a proteolytic system involving a family of proteases called cystein aspartic acid-specific proteases (caspases) (147, 148). The biochemical and cellular hallmarks of apoptosis is characterized by nuclear and DNA fragmentation and condensation, membrane blebbing, and cellular shrinkage. Although a fully comprehensive model of apoptosis has not been assembled, two general pathways have been delineated (45, 139, 148, 165), namely, the intrinsic or mitochondrial pathway and the extrinsic or DR pathway. In the intrinsic pathway, apoptosis occurs when specific stimuli lead to mitochondrial release of cytochrome c. Cytochrome c binds apoptotic protease-activating factor-1 in the cytosol and induces its oligomerization and the subsequent recruitment and activation of procaspase-9. In the DR pathway, a ligand such as CD95L/Fas ligand binding to DR leads to the activation of caspase-8 through specific adaptor molecules such as FADD. Both caspase-8 and -9 can lead to the activation of caspase-3 and to ultimate cell death. The two pathways coexist in many circumstance (29, 30, 87) and in cardiomyocytes (14, 67, 93, 143, 159).
TLR signaling modulates cardiomyocyte apoptosis.
TLR4.
TLR4 has been linked to both parallel proapoptotic and survival pathways. LPS induces apoptosis in endothelial cells (27) and hepatocytes (49), whereas it has an antiapoptotic effect in monocytes (44, 94), neutrophils (156), macrophages (90), and cardiomyocytes (26, 170). In the cells protected by LPS treatment, the activation of TLR4 triggers the expression of cell survival and inflammatory genes via NF-κB-dependent mechanisms. In the heart, an early report indicated that prolonged LPS treatment in rats activated proapoptotic and survival pathways and induced very modest cardiomyocyte apoptosis (100). However, the low levels of apoptosis appeared insufficient to account for the LPS-induced cardiomyocyte dysfunction, and its significance is uncertain. The data from a septic animal model (cecal ligation and puncture) indicated a minimal level of apoptosis in the heart, whereas a significant level of apoptosis was found in lymphocytes and in parenchymal cells of ileum, colon, lung, and skeletal muscle (33). Autopsy studies from patients who died of sepsis revealed minor apoptotic cell death in the heart, whereas there was profound lymphocyte and gastrointestinal epithelial cell death (56–58). In fact, a recent study indicated that an in vivo administration of LPS actually reduced myocardial apoptosis induced by I/R injury (48). Moreover, in cell types such as endothelial cells, the induction of LPS-induced apoptosis did not occur unless the production of endogenous survival proteins was blocked (10, 11), again suggesting a parallel survival pathways in these cells. In an in vitro model of apoptosis, recent studies have demonstrated that LPS leads to a time- and dose-dependent antiapoptotic effect and phosphorylation of Akt and ERK in isolated cardiomyocytes (26). Both TLR4 and its adaptor MyD88 seem to mediate the survival benefit in the LPS-treated cardiomyocytes (170). The LPS-induced antiapoptotic effect can be blocked by phosphatidylinositol 3-kinase (PI3-kinase) inhibitor, MEK1 inhibitor, and by dnIKK-β, suggesting that all three kinases, i.e., Akt, ERK, and IKK-β, contribute to the LPS-induced survival effect in cardiomyocytes (26). Moreover, TLR4 mediates a robust inducible nitric oxide (NO) synthase (iNOS) induction both in mouse hearts and in isolated cardiomyocytes subjected to LPS. In that study (170), the antiapoptotic effect of LPS was almost abolished in cardiomyocytes deficient of iNOS, suggesting that iNOS was essential for the TLR4-induced antiapoptotic effect (26). It is unclear and of great interest as to how endogenous iNOS regulates, in response to TLR4 stimulation, apoptosis signaling. It is noteworthy that iNOS also appears essential for the other form of cardioprotection, i.e., ischemic preconditioning (47), and that NO, derived from NO donors, has been shown to inhibit caspase-3 activity through S-nitrosylation and to reduce cardiomyocyte apoptosis (92, 129). However, the role of endogenous iNOS-NO in response to TLR4 activation in regulating caspase activity in cardiomyocytes has yet to be determined.
TLR2.
An early study indicated that the synthetic lipopeptide or Escherichia coli lipoprotein induced NF-κB activation and apoptosis in 293 cells (human embryonic kidney cells) that were stably transfected with human TLR2 but not in parental 293 cells which did not express TLR2, suggesting that TLR2 mediated a proapoptosis signaling (2). TLR2 also induces apoptosis in a human acute monocyte leukemia cell line THP-1 and in epithelial cells (2, 3). By contrast, other investigators have suggested that TLR2 signaling mediates an antiapoptotic effect and a proinflammatory pathway in cardiomyocytes subjected to hydrogen peroxide (40). These investigators found that oxidative stress-induced cytotoxicity in neonatal rat cardiomyocytes was enhanced by blocking TLR2 signaling with a specific antagonistic antibody. The reason for the discrepancy is unclear but likely mutilfactorial. The final phenotypic outcome of TLR2 activation with respect to cell survival may well be dependent on cell types (cell lines vs. primary cultures) and the type of apoptosis models and agents used.
MyD88.
In the transfected 293 cell line, the expression of high levels of exogenous MyD88 mediated TLR2-induced apoptosis, possible through FADD-caspase-8-dependent pathway (3). However, using a genetically engineered mice (MyD88−/−) and in an acute lung injury model, Jiang et al. (68) have recently demonstrated that MyD88 mediates an antiapoptotic signal in lung epithelial cells and plays a critical role in pulmonary tissue repair and inflammation during the bleomycin-induced lung injury. Similarly, MyD88 appears essential for TLR4-activated antiapoptotic signaling in mouse cardiomyocytes (170) and in macrophages (90). Moreover, the adenovirus-mediated expression of MyD88 modulates TLR2-induced cytokine production in mouse cardiomyocytes (169) but is not sufficient to affect cardiomyocyte apoptosis in vitro. By contrast, others reported that an overexpression of dnMyD88 led to reduced cardiomyocyte apoptosis in an in vivo model of I/R injury (59).
Interestingly, myocardial IRAK-1, a kinase critical for TLR signaling, quickly becomes activated in response to ischemic injury (26). It is unclear, however, as to how the kinase activation is initiated upon myocardial ischemia and the biological significance that it may confer. Importantly, transgene expression of IRAK-1, but not its kinase-deficient mutant, appears sufficient to protect cardiomyocytes against apoptosis in vitro (26).
LPS Preconditioning Against I/R Injury
Evidence from several lines of investigation suggests that the activation of systemic TLR4 by LPS protects myocardium against I/R injury (Table 1). In animal models of I/R injury (13, 18, 48, 88, 89, 99, 106, 138, 155, 166, 167), in both in vivo (13, 48, 99, 138, 155, 166, 167) and ex vivo (18, 88, 106), a prior systemic administration of a sublethal dose of LPS reduces subsequent MI and improved cardiac functions. For example, hearts isolated from rats pretreated with a low dose of LPS (0.5 mg/kg) 24 h before had a better preserved myocardial function after I/R compared with the saline-treated control hearts (18, 105). Similar cardiac protection in LPS-treated animals was observed in vivo and in different animal models of I/R injury, such as rabbit (13, 99), rat (18, 88, 106, 138, 155, 166, 167), and mice (48). The cardioprotective effect of LPS usually occurs between 12–24 h after the administration of LPS and is abolished by cycloheximide (106), suggesting a mechanism involving the de novo synthesis of cardioprotective proteins. LPS administration induces a robust induction of iNOS in the heart (154, 170), a process mediated via TLR4 (170). Similar to ischemic preconditioning (16), the cardioprotection conferred by LPS seems to be mediated by iNOS (155) and by Akt (48). Ha and colleagues (48) recently demonstrated that the cardiac benefit of LPS against I/R injury was abolished by an intraperitoneal administration of a PI3-kinase inhibitor or in transgenic mice expressing the inactive Akt mutant. These data suggest that LPS exhibits its cardioprotection via PI3-kinase/Akt pathway and appear to be consistent with the in vitro observation that Akt, among other survival pathways, mediates a TLR4-mediated antiapoptotic benefit (26, 170). The aforementioned studies are important since they demonstrated a clear link between the activation of systemic TLR4 innate immune system and cardioprotection against ischemic myocardial injury and are consistent with the in vitro findings in isolated cardiomyocytes that demonstrate the antiapoptotic property of TLR4 signaling. However, given the multiple systemic responses following an in vivo administration of LPS, it is unclear whether the observed cardiac benefits are the direct results of TLR4 signaling or due to other events secondary to systemic TLR4 activation. Therefore, the critical role of TLR4, particularly that of cardiac origin, in the cardioprotection in vivo remains to be defined.
Table 1.
LPS-induced preconditioning against ischemic myocardial injury
| Cardiac I/R Models | Species | Effects on the Heart | References |
|---|---|---|---|
| Ex vivo | |||
| I/R | Rat | Increased LVDP | 18,106 |
| Increased ±dP/dtmax | |||
| Neutrophil-mediated I/R | Rat | Increased LVDP | 88 |
| Increased ±dP/dtmax | |||
| In vivo | |||
| Rat | Reduced ventricular arrhythmias and infarct size | 138 | |
| Rat | Reduced infarct sizes; decreased cardiac troponin T | 166,167 | |
| Rat | Reduced infarct sizes via iNOS induction | 155 | |
| Rabbit | Reduced infarct sizes | 13,99 | |
| Mouse | Reduced infarct sizes via PI3-kinase/Akt | 48 |
I/R, ischemia-reperfusion; LVDP, left ventricular (LV) developed pressure; +dP/dtmax, maximum first derivative of developed LV pressure; −dP/dtmax, minimum first derivative of developed LV pressure; iNOS, inducible nitric oxide synthase; PI3-kinase, phosphatidylinositol 3-kinase.
TLR Signaling Mediates Myocardial I/R Injury
Innate immune response during myocardial I/R.
The innate immune response to I/R is, by far, the most common cause of myocardial inflammation. Acute inflammation is a complex response of soluble and cellular factors that together serve as the effector mechanisms of innate immunity. Molecular and cellular mechanisms underlying I/R injury are complex (164), involving the activation of endothelial cells and complement, an increased vascular permeability, and a rapid accumulation of neutrophils (38, 110, 119). Several hours after the onset of myocardial reperfusion, neutrophils accumulate in the infarcted myocardial tissue in response to the release of chemoattractants: reactive oxygen species (ROS), cytokines, and the activated complements. The upregulated cell-adhesion molecules (ICAM-1, VCAM-1, and P-selectin), likely mediated by NF-κB activation (86) in response to myocardial ischemia, facilitate the migration of neutrophils into the myocardial tissue. Neutrophils mediate cardiomyocyte death by causing vascular plugging, releasing degradative enzymes, and ROS (152), which causes myocardial injury by inducing a mitochondrial permeability transition pore opening and a subsequent ATP depletion and cell death (50). Although many of the downstream events leading to I/R injury as mentioned above have been identified, the proximal signaling mechanisms that control these critical events during I/R remain incompletely defined.
Inflammation contributes to I/R injury.
Evidence from several lines of investigation suggests that inflammation is an important functional contributor to the pathogenesis of ischemic myocardial injury. For example, in animal models, neutrophil depletion with antibodies (128) or physical filtering (34, 61), as well as the inhibition of neutrophil adhesion with anti-CD18 monoclonal antibody (142), all substantially reduce injury after reperfusion. In addition, interventions targeted at a variety of specific inflammatory mediators have demonstrated benefits in I/R injury, including complement depletion (96) or lipoxygenase inhibitors (133), or antibodies to the proinflammatory cytokine IL-1 (60). However, some anti-inflammatory intervention, notably corticosteroids, have yielded disappointing results and actually increased infarct size and its complications (126). Interestingly, some investigators have suggested that inflammation may actually play a beneficial role in the healing process after infarction (77). It is likely that inflammation may play multiple roles in injury and recovery after I/R injury. For example, early neutrophil infiltration may contribute to injury but later, predominantly mononuclear leukocyte recruitment may represent an important part of the healing process.
It is noteworthy that I/R injury can also occur experimentally in isolated hearts perfused with crystalloid solutions lacking cellular blood components and other systemic contributors (51) (e.g., in Langendorff perfusion system), as well as in the heart perfused with whole blood (e.g., in vivo I/R injury models). The common feature under both conditions is the reintroduction of molecular oxygen, which contributes to the production of oxygen free radicals that cause tissue injury. Under conditions of whole blood reperfusion, there is the added influence of inflammatory cells such as neutrophils, as well as other blood-borne components. In addition, whole blood reperfusion in vivo also involves the activation of the coagulation cascade and the formation of the thrombolic occlusion of vessels and heterogeneity in the distribution of blood flow within the area of risk.
TLRs mediate myocardial inflammation and injury during I/R.
Frantz and colleagues (41) first documented that there was an enhanced TLR4 expression in remodeling murine myocardium remote from sites of ischemic injury and in heart tissue from patients with idiopathic dilated cardiomyopathy. What is unclear, however, is the functional significance of TLR4 upregulation under the cardiac conditions. During the past five years, evidence has emerged that clearly indicates that in addition to its role in the host immunity against invading pathogens, TLR signaling may also play a critical role in modulating cell survival and tissue injury (or repair) in “noninfectious” injury models in several organs, such as lung (68), liver (121), brain (84, 144), and heart (28, 118, 136, 149), although, to make things even more complicated, TLR signaling seems to have different roles in different injury models. Of note, all of these investigations have been carried out in mouse models where genetic modifications of target genes are readily available. For example, in the bleomyocin-induced acute lung injury model (68), TLR2 and TLR4 seemed to mediate proinflammatory and prosurvival signaling. Hyaluronan, produced in response to the acute lung injury, induces a proinflammatory and an antiapoptotic effect in lung epithelial cells via both TLR2- and TLR4-MyD88-dependent mechanisms (68). Similarly, in a hyperoxic lung injury model, the lack of TLR4 led to an increase in lung injury, apoptosis, and mortality (168), whereas the induced, active TLR4 (CD4hTLR4) transgenic expression in the lung confers resistance to hyperoxia-induced pulmonary apoptosis (124). By contrast, mice with the inactive TLR4 mutant or genetically deficient for TLR4 (28, 75, 118), TLR2 (35), or MyD88 (36) or pretreated intravenously with a TLR4 antagonist (Eritoran) (134) exhibited reduced MI sizes compared with WT or vehicle-treated animals, respectively, suggesting that TLR2 and TLR4 signaling contributed to ischemic injury in the heart (Table 2). However, the in vivo studies may have been complicated by the systemic deficiency/inhibition of TLR signaling. The lack of TLR signaling in extra-cardiac sources such as inflammatory cells could have contributed to the reduction of myocardial inflammation and thus injury after I/R. Recent studies have supported the notion that TLR4 of the extra-cardiac source (i.e., bone marrow-derived hematopoietic cells) plays an important role in mediating cardiac dysfunction under certain pathological conditions (145). In fact, in all of these studies mentioned above, a systemic TLR deficiency also leads to a significant reduction in the level of myocardial inflammation as measured by neutrophil recruitment, an NF-κB-dependent expression of cytokines and chemokines, and a complement deposition in the heart after I/R. Therefore, it seems possible that the reduction in myocardial inflammation may have contributed to reduced MI sizes in these TLR-deficient animals. Thus the exact role of cardiac (vs. bone marrow-derived inflammatory cell) TLRs in ischemic myocardial injury remains to be investigated.
Table 2.
TLR signaling modulates myocardial I/R injury and remodeling
| Mice | Infarct Models | Effects in Knockout Mice | References |
|---|---|---|---|
| TLR2−/− | |||
| I/R (30′ I/60 R′) | Smaller infarct sizes, reduced neutrophil recruitment, reduced ROS and cytokines | 35 | |
| Permanent coronary ligation | Improved survival rate, attenuated remodeling, but same infarct sizes at 4 wk | 136 | |
| TLR4 | |||
| C57 BL/10 ScCr C3H/HeJ | I/R (60′ I/24 h R) | Smaller infarct sizes, reduced MPO activity and complement 3 deposition | 118 |
| C3H/HeJ | I/R (60′ I/120′ R) | Smaller infarct sizes, decreased cardiac expression of TNF, MCP-1, and ILs | 28 |
| C3H/HeJ | I/R (60′ I/24 h R) | Smaller infarct sizes, but no gain in LV function | 75 |
| WT with eritoran | I/R (30′ I/120′ R) | Smaller infarct sizes, reduced pJNK, reduced cytokine expression | 134 |
| C3H/HeJ | Permanent coronary ligation | Reduced LV remodeling, improved systolic function, reduced cytokine expression | 149 |
| C57 BL/10 ScCr | Permanent coronary ligation | Improved LV function on day 6 after infarction, improved survival rate, reduced LV remodeling and apoptosis at 4 wk. | 125 |
| MyD88−/− | I/R (30′ I/24 h R) | Smaller infarct sizes, improved LV function, and attenuated cytokine expression and neutrophil recruitment | 36 |
TLR, Toll-like receptor; ROS, reactive oxygen species; MPO, myeloperoxidase; MCP-1, monocyte chemoattractant protein-1; pJNK, phosphorylated JNK; MyD88, myeloid differentiation primary-response gene 88.
One of the key questions with respect to the role of TLR signaling in myocardial I/R injury is if and how TLRs become activated in response to ischemia and reperfusion. The observation that myocardial IRAK-1 quickly becomes activated following coronary artery ligation seems to suggest that TLRs (or IL-1) respond to ischemic insult, although the upstream signaling remains unclear (26). Over the past 10 years, using the cells derived from TLR2−/− or TLR4 mutant mice or cells transfected with TLRs, investigators have identified a numbers of endogenous mediators that induce proinflammatory cytokine production through TLR2- and/or TLR4-dependent manners (Table 3). For example, in addition to LPS, TLR4 recognizes heat shock proteins (Hsp-22, Hsp-60, and Hsp-70) (6, 31, 113, 127, 150, 151), fibrinogen (137), fibronectin containing repeat extra domain A (114), surfactant protein-A (46), and soluble heparan sulfate (70). TLR2 is known to recognize Hsp-60 and Hsp-70 (6, 150, 151) and hyaluronan (68). Some of these endogenous molecules such as Hsp-60 and Hsp-70 are produced in response to myocardial ischemia (78, 95) and confer potent antiapoptotic and cardioprotective effects in the heart (66, 68, 76, 115). It remains unclear, however, and would be of great importance to investigate whether or not TLRs mediate the cardiac benefit induced by these molecular chaperons and how to reconcile these findings with the suggested role of TLRs in I/R injury in the aforementioned loss-of-function studies.
Table 3.
TLRs and their endogenous ligands
| TLRs | Endogenous Ligands | Cells | End Points | References |
|---|---|---|---|---|
| TLR2 | Hsp-60 | Fibroblasts | P38, JNK1/2, ERK1/2, IKK | 150 |
| Hsp-70 | HEK293 | NF-κB | 6 | |
| Fibroblasts | NF-κB | 151 | ||
| Hyaluronan | Macrophages | MIP-2, TNF, KC | 68 | |
| TLR4 | Hsp-22 | Macrophages | IL-6 | 127 |
| Hsp-60 | Fibroblasts | P38, JNK1/2, ERK1/2, IKK | 150 | |
| Macrophages | TNF, NO | 113 | ||
| Hsp-70 | HEK293 | NF-κB | 6 | |
| Fibroblasts | NF-κB | 151 | ||
| Macrophage | TNF | 31 | ||
| Fibronectin (extra domain A) | HEK293 | NF-κB | 114 | |
| Fibrinogen | Macrophages | MCP-1 | 137 | |
| Hyaluronic acid (oligosaccharides) | Dendritic cells | TNF, pMAPK | 146 | |
| NF-κB | ||||
| Heparan sulfate (polysaccharide) | Dendritic cells | DC maturation | 70 | |
| Hyaluronan | Macrophages | MIP-2, TNF, KC | 68 | |
| Lung surfactant protein A | Macrophages | NF-κB activity | 46 | |
| Ovary cells | TNF, IL-10 |
Hsp, heat shock protein; KC, keratinocyte chemoattractant; NO, nitric oxide; MCP-1, monocyte chemoattractant protein-1; pMAPK, phosphorylated MAPK; DC, dendritic cell; MIP-2, macrophage inflammatory protein-2.
MyD88, a key adaptor, contributes to myocardial I/R injury.
As illustrated in Fig. 1, MyD88 is a key adaptor protein that is critical for transducing signals from all TLR family members (64, 73, 104, 108), except TLR3, and IL-1 receptor family members. Recent studies, employing adenovirus-mediated transgene expression and genetically modified mice, have demonstrated that MyD88 may play an important role in the pathogenesis of I/R injury in the heart. When overexpressed in the heart, dnMyD88 led to a reduction in MI size and apoptosis in a rat model of I/R injury (59). It is of interest that the transgene expression of dnMyD88 also led to a decrease in myocardial inflammation given the well-characterized host innate immune response to adenoviral vectors in the heart (122). In a mouse model of I/R injury, Feng and colleagues (36) recently found that mice deficient of MyD88 had a reduced neutrophil recruitment and attenuated cytokine/adhesion molecule production (keratinocyte chemoattractant, monocyte chemoattractant protein-1, and ICAM-1), smaller infarct sizes, and much better preserved ventricular function as demonstrated by serial transthoracic echocardiographic and invasive hemodynamic measurements. In an effort to determine the potential impact of MyD88 deficiency in circulatory components on cardiac injury associated with the in vivo model of I/R, they tested the impact of cardiac MyD88 deficiency on myocardial injury in isolated mouse hearts. Surprisingly, they found that in isolated hearts subjected to global I/R, MyD88 deficiency had no effect on MI and left ventricular (LV) function following I/R (36). This finding suggests that in an in vivo condition, MyD88 may play a pivotal role in mediating myocardial inflammation that is critical for I/R injury and is consistent with the hypothesis that the cardiac benefits observed in MyD88−/− mice in vivo may require circulating blood components during I/R.
TLR signaling modulates LV remodeling after ischemic injury.
LV remodeling represents myocardial structural changes in the LV in response to chronic alterations in loading conditions and contribute to morbidity and mortality after ischemic injury (117). LV remodeling can be induced by either pressure overload or volume overload, which results in a concentric remodeling or an eccentric hypertrophy, respectively. After MI, a stretched and dilated infarcted tissue increases LV volume with a combined volume and pressure load on noninfarcted areas. In a permanent coronary artery ligation model, Shishido and colleagues (136) demonstrated that TLR2-deficient mice had less LV remodeling at 4 wk, improved LV function at 1 and 4 wk, and a significantly higher survival rate at 4 wk compared with WT mice (Table 2). The reduced LV remodeling was demonstrated by a decreased myocardial fibrosis in the noninfarcted areas, a reduced transforming growth factor-β, a collagen type 1 mRNA expression, and small LV dimensions at end diastole. Interestingly, the infarct size (at 4 wk) and level of inflammation (at 3–7 days) were similar between the two groups of animals in this model of MI. It is noteworthy, however, that the investigators in that study did not measure the infarct sizes during the early stage (e.g., 24–48 h) of ischemic injury. Therefore, the possibility that a potential MI size reduction in TLR2−/− mice compared with WT mice may contribute to the attenuated myocardial remodeling in TLR2−/− animals needs to be ruled out. In a similar model of postinfarction remodeling, TLR4 was found to modulate survival as well as LV remodeling after ischemic injury (125, 149). Although an enhanced JNK expression has been implicated as a possible intracellular mechanism for the attenuated LV remodeling in TLR4−/− mice (125), the exact mechanisms as to how TLR2 and TLR4 signaling is initiated during myocardial ischemia and how they modulate subsequent ventricular remodeling remain elusive.
Summary
During the past decade, emerging evidence has indicated that in addition to their critical role in host immunity against microbial infection, TLRs can also function as a sensor responding to tissue stress such as hypoxia and ischemia and modulate cell survival and tissue injury. Several lines of investigation have demonstrated the importance of cardiomyocyte apoptosis in myocardial I/R injury. Existing data suggest that TLR2 and TLR4 may mediate both pro- and antiapoptotic signaling, and the final phenotypic outcome probably depends on the cell types and the model of apoptosis used, although recent studies, both in vitro and in vivo, appear to support the notion that a direct activation of TLR4 leads to an antiapoptotic signaling in cardiomyocytes. A large body of evidence in several animal models demonstrates that LPS, at sublethal doses, has a preconditioning-like effect protecting the heart against subsequent I/R injury. However, given the multiple systemic reactions in response to an in vivo administration of LPS in these studies, it is unclear whether the observed cardiac benefits are the direct result of TLR4 stimulation or due to other events secondary to systemic TLR4 innate immune activation. Therefore, the critical role of TLR4, particularly that of cardiac origin, in the cardioprotection in vivo is still unclear. By contrast and somewhat paradoxically, studies from several laboratories using knockout mouse models suggest that TLR2, TLR4, and MyD88 may all contribute to myocardial inflammation and infarction after I/R. It is noteworthy, however, that in these models, TLR and MyD88 deficiency are systemic and, therefore, the exact contribution of TLR signaling of cardiac (vs. circulatory) origin is unknown. Nevertheless, the disparate findings illustrate the complexity and difficulties in defining the emerging role of TLRs as a critical modulator in both tissue inflammation and injury. Defining the role of innate immune signaling in ischemic myocardial injury may have important therapeutic implications.
GRANTS
This work was supported by the Foundation for Anesthesia Education and Research and American Society of Anesthesiologists, an American Heart Association Grant 0755890T, The William Milton Foundation of Harvard University, and National Institutes of Health Grants HL-04336 and GM-080906.
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285: 736–739, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis AO, Yang RB, Weiss DS, Godowski P, Zychlinsky A. The apoptotic signaling pathway activated by Toll-like receptor-2. EMBO J 19: 3325–3336, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KV, Bokla L, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell 42: 791–798, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42: 779–789, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11: 255–260, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 281: 1305–1308, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Balistreri CR, Candore G, Colonna-Romano G, Lio D, Caruso M, Hoffmann E, Franceschi C, Caruso C. Role of Toll-like receptor 4 in acute myocardial infarction and longevity. JAMA 292: 2339–2340, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Bannerman DD, Tupper JC, Erwert RD, Winn RK, Harlan JM. Divergence of bacterial lipopolysaccharide pro-apoptotic signaling downstream of IRAK-1. J Biol Chem 277: 8048–8053, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Bannerman DD, Tupper JC, Ricketts WA, Bennett CF, Winn RK, Harlan JM. A constitutive cytoprotective pathway protects endothelial cells from lipopolysaccharide-induced apoptosis. J Biol Chem 276: 14924–14932, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol 270: 81–92, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Belosjorow S, Schulz R, Dorge H, Schade FU, Heusch G. Endotoxin and ischemic preconditioning: TNF-α concentration and myocardial infarct development in rabbits. Am J Physiol Heart Circ Physiol 277: H2470–H2475, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Bialik S, Cryns VL, Drincic A, Miyata S, Wollowick AL, Srinivasan A, Kitsis RN. The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ Res 85: 403–414, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Boekholdt SM, Agema WR, Peters RJ, Zwinderman AH, van der Wall EE, Reitsma PH, Kastelein JJ, Jukema JW. Variants of toll-like receptor 4 modify the efficacy of statin therapy and the risk of cardiovascular events. Circulation 107: 2416–2421, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Bolli R Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292: H19–H27, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyocytes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res 72: 384–393, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Brown JM, Grosso MA, Terada LS, Whitman GJ, Banerjee A, White CW, Harken AH, Repine JE. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci USA 86: 2516–2520, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med 197: 263–268, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science 271: 1128–1131, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature 383: 443–446, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol 7: 1074–1081, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Chao W, Matsui T, Novikov MS, Tao J, Li L, Liu H, Ahn Y, Rosenzweig A. Strategic advantages of insulin-like growth factor-I expression for cardioprotection. J Gene Med 5: 277–286, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Chao W, Shen Y, Li L, Rosenzweig A. Importance of FADD signaling in serum deprivation- and hypoxia-induced cardiomyocyte apoptosis. J Biol Chem 277: 31639–31645, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Chao W, Shen Y, Novikov MS, Li L, Ahn Y, Rosenzweig A. Adenoviral expression of dominant-negative FADD blocks cardiomyocyte apoptosis and reduces myocardial injury after transient ischemia (Abstract). Circulation 106: II–131, 2002. [Google Scholar]
- 26.Chao W, Shen Y, Zhu X, Zhao H, Novikov M, Schmidt U, Rosenzweig A. Lipopolysaccharide improves cardiomyocyte survival and function after serum deprivation. J Biol Chem 280: 21997–22005, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Choi KB, Wong F, Harlan JM, Chaudhary PM, Hood L, Karsan A. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem 273: 20185–20188, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Chong AJ, Shimamoto A, Hampton CR, Takayama H, Spring DJ, Rothnie CL, Yada M, Pohlman TH, Verrier ED. Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg 128: 170–179, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev 16: 33–45, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell 115: 61–70, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation 105: 685–690, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Edfeldt K, Bennet AM, Eriksson P, Frostegard J, Wiman B, Hamsten A, Hansson GK, de Faire U, Yan ZQ. Association of hypo-responsive toll-like receptor 4 variants with risk of myocardial infarction. Eur Heart J 25: 1447–1453, 2004. [DOI] [PubMed] [Google Scholar]
- 33.enchHotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med 25: 1298–1307, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Engler RL, Dahlgren MD, Morris DD, Peterson MA, Schmid-Schonbein GW. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol Heart Circ Physiol 251: H314–H323, 1986. [DOI] [PubMed] [Google Scholar]
- 35.Favre J, Musette P, Douin-Echinard V, Laude K, Henry JP, Arnal JF, Thuillez C, Richard V. Toll-like receptors 2-deficient mice are protected against postischemic coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol 27: 1064–1071, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Zhao H, Xu X, Buys ES, Raher MJ, Bopassa JC, Thibault H, Scherrer-Crosbie M, Schmidt U, Chao W. Innate immune adaptor MyD88 mediates neutrophil recruitment and myocardial injury after ischemia-reperfusion in mice. Am J Physiol Heart Circ Physiol 295: H1311–H1318, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O'Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413: 78–83, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med 4: 444–454, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem 276: 5197–5203, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 104: 271–280, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94: 1621–1628, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goyal A, Wang Y, Graham MM, Doseff AI, Bhatt NY, Marsh CB. Monocyte survival factors induce Akt activation and suppress caspase-3. Am J Respir Cell Mol Biol 26: 224–230, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Green DR, Reed JC. Mitochondria and apoptosis. Science 281: 1309–1312, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves Toll-like receptor 4. J Immunol 168: 5989–5992, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA 96: 11507–11512, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischaemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovasc Res 78: 546–553, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Hamada E, Nishida T, Uchiyama Y, Nakamura J, Isahara K, Kazuo H, Huang TP, Momoi T, Ito T, Matsuda H. Activation of Kupffer cells and caspase-3 involved in rat hepatocyte apoptosis induced by endotoxin. J Hepatol 30: 807–818, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Hausenloy DJ, Yellon DM. The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol 35: 339–341, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Hearse DJ, Humphrey SM, Chain EB. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol 5: 395–407, 1973. [DOI] [PubMed] [Google Scholar]
- 52.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J Jr, Muller W, Chien KR. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 97: 189–198, 1999. [DOI] [PubMed] [Google Scholar]
- 53.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424: 743–748, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Holloway JW, Yang IA, Ye S. Variation in the toll-like receptor 4 gene and susceptibility to myocardial infarction. Pharmacogenet Genomics 15: 15–21, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162: 3749–3752, 1999. [PubMed] [Google Scholar]
- 56.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Hotchkiss RS, Schmieg RE Jr, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Karl IE, Buchman TG. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit Care Med 28: 3207–3217, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27: 1230–1251, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Hua F, Ha T, Ma J, Gao X, Kelley J, Williams DL, Browder IW, Kao RL, Li C. Blocking the MyD88-dependent pathway protects the myocardium from ischemia/reperfusion injury in rat hearts. Biochem Biophys Res Commun 338: 1118–1125, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Hwang MW, Matsumori A, Furukawa Y, Ono K, Okada M, Iwasaki A, Hara M, Miyamoto T, Touma M, Sasayama S. Neutralization of interleukin-1beta in the acute phase of myocardial infarction promotes the progression of left ventricular remodeling. J Am Coll Cardiol 38: 1546–1553, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Ito BR, Engler RL, del Balzo U. Role of cardiac mast cells in complement C5a-induced myocardial ischemia. Am J Physiol Heart Circ Physiol 264: H1346–H1354, 1993. [DOI] [PubMed] [Google Scholar]
- 62.Itoh G, Tamura J, Suzuki M, Suzuki Y, Ikeda H, Koike M, Nomura M, Jie T, Ito K. DNA fragmentation of human infarcted myocardial cells demonstrated by the nick end labeling method and DNA agarose gel electrophoresis. Am J Pathol 146: 1325–1331, 1995. [PMC free article] [PubMed] [Google Scholar]
- 63.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell 11: 293–302, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci 27: 474–482, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol 12: 467–471, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Jayakumar J, Suzuki K, Sammut IA, Smolenski RT, Khan M, Latif N, Abunasra H, Murtuza B, Amrani M, Yacoub MH. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation 104: I303–I307, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Jeremias I, Kupatt C, Martin-Villalba A, Habazettl H, Schenkel J, Boekstegers P, Debatin KM. Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation 102: 915–920, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol 22: 7158–7167, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168: 5233–5239, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest 74: 86–107, 1996. [PubMed] [Google Scholar]
- 72.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11: 115–122, 1999. [DOI] [PubMed] [Google Scholar]
- 73.Kawai T, Akira S. TLR signaling. Cell Death Differ 13: 816–825, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol 167: 5887–5894, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Kim SC, Ghanem A, Stapel H, Tiemann K, Knuefermann P, Hoeft A, Meyer R, Grohe C, Knowlton AA, Baumgarten G. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol 7: 5, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation 105: 2899–2904, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Kloner RA, Fishbein MC, Lew H, Maroko PR, Braunwald E. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation 57: 56–63, 1978. [DOI] [PubMed] [Google Scholar]
- 78.Knowlton AA, Brecher P, Apstein CS. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest 87: 139–147, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knuefermann P, Nemoto S, Misra A, Nozaki N, Defreitas G, Goyert SM, Carabello BA, Mann DL, Vallejo JG. CD14-deficient mice are protected against lipopolysaccharide-induced cardiac inflammation and left ventricular dysfunction. Circulation 106: 2608–2615, 2002. [DOI] [PubMed] [Google Scholar]
- 80.Knuefermann P, Sakata Y, Baker JS, Huang CH, Sekiguchi K, Hardarson HS, Takeuchi O, Akira S, Vallejo JG. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation 110: 3693–3698, 2004. [DOI] [PubMed] [Google Scholar]
- 81.Knuefermann P, Schwederski M, Velten M, Krings P, Ehrentraut H, Rudiger M, Boehm O, Fink K, Dreiner U, Grohe C, Hoeft A, Baumgarten G, Koch A, Zacharowski K, Meyer R. Bacterial DNA induces myocardial inflammation and reduces cardiomyocyte contractility: role of toll-like receptor 9. Cardiovasc Res 78: 26–35, 2008. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110: 191–202, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Koch W, Hoppmann P, Pfeufer A, Schomig A, Kastrati A. Toll-like receptor 4 gene polymorphisms and myocardial infarction: no association in a Caucasian population. Eur Heart J 27: 2524–2529, 2006. [DOI] [PubMed] [Google Scholar]
- 84.Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol 190: 28–33, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86: 973–983, 1996. [DOI] [PubMed] [Google Scholar]
- 86.Li C, Browder W, Kao RL. Early activation of transcription factor NF-κB during ischemia in perfused rat heart. Am J Physiol Heart Circ Physiol 276: H543–H552, 1999. [DOI] [PubMed] [Google Scholar]
- 87.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94: 491–501, 1998. [DOI] [PubMed] [Google Scholar]
- 88.Lipton BP, Bautista AP, Delcarpio JB, McDonough KH. Effects of endotoxin on neutrophil-mediated I/R injury in isolated perfused rat hearts. Am J Physiol Heart Circ Physiol 280: H802–H811, 2001. [DOI] [PubMed] [Google Scholar]
- 89.Lipton BP, Delcarpio JB, McDonough KH. Effects of endotoxin on neutrophil-mediated ischemia/reperfusion injury in the rat heart in vivo. Exp Biol Med (Maywood) 226: 320–327, 2001. [DOI] [PubMed] [Google Scholar]
- 90.Lombardo E, Alvarez-Barrientos A, Maroto B, Bosca L, Knaus UG. TLR4-mediated survival of macrophages is MyD88 dependent and requires TNF-alpha autocrine signalling. J Immunol 178: 3731–3739, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Lord KA, Hoffman-Liebermann B, Liebermann DA. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene 5: 1095–1097, 1990. [PubMed] [Google Scholar]
- 92.Maejima Y, Adachi S, Morikawa K, Ito H, Isobe M. Nitric oxide inhibits myocardial apoptosis by preventing caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol 38: 163–174, 2005. [DOI] [PubMed] [Google Scholar]
- 93.Malhotra R, Brosius FC 3rd. Glucose uptake and glycolysis reduce hypoxia-induced apoptosis in cultured neonatal rat cardiac myocytes. J Biol Chem 274: 12567–12575, 1999. [DOI] [PubMed] [Google Scholar]
- 94.Manna SK, Aggarwal BB. Lipopolysaccharide inhibits TNF-induced apoptosis: role of nuclear factor-kappaB activation and reactive oxygen intermediates. J Immunol 162: 1510–1518, 1999. [PubMed] [Google Scholar]
- 95.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation 88: 1264–1272, 1993. [DOI] [PubMed] [Google Scholar]
- 96.Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, Hale SL. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest 61: 661–670, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation 100: 2373–2379, 1999. [DOI] [PubMed] [Google Scholar]
- 98.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001. [DOI] [PubMed] [Google Scholar]
- 99.Mazenot C, Gobeil F, Ribuot C, Regoli D, Godin-Ribuot D. Delayed myocardial protection induced by endotoxin does not involve kinin B(1)-receptors. Br J Pharmacol 131: 740–744, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McDonald TE, Grinman MN, Carthy CM, Walley KR. Endotoxin infusion in rats induces apoptotic and survival pathways in hearts. Am J Physiol Heart Circ Physiol 279: H2053–H2061, 2000. [DOI] [PubMed] [Google Scholar]
- 101.Medzhitov R Toll-like receptors and innate immunity. Nat Rev Immunol 1: 135–145, 2001. [DOI] [PubMed] [Google Scholar]
- 102.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med 343: 338–344, 2000. [DOI] [PubMed] [Google Scholar]
- 103.Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397, 1997. [DOI] [PubMed] [Google Scholar]
- 104.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2: 253–258, 1998. [DOI] [PubMed] [Google Scholar]
- 105.Meng X, Ao L, Brown JM, Fullerton DA, Banerjee A, Harken AH. Nitric oxide synthase is not involved in cardiac contractile dysfunction in a rat model of endotoxemia without shock. Shock 7: 111–118, 1997. [DOI] [PubMed] [Google Scholar]
- 106.Meng X, Ao L, Brown JM, Meldrum DR, Sheridan BC, Cain BS, Banerjee A, Harken AH. LPS induces late cardiac functional protection against ischemia independent of cardiac and circulating TNF-α. Am J Physiol Heart Circ Physiol 273: H1894–H1902, 1997. [DOI] [PubMed] [Google Scholar]
- 107.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock 26: 430–437, 2006. [DOI] [PubMed] [Google Scholar]
- 108.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278: 1612–1615, 1997. [DOI] [PubMed] [Google Scholar]
- 109.Nemoto S, Vallejo JG, Knuefermann P, Misra A, Defreitas G, Carabello BA, Mann DL. Escherichia coli LPS-induced LV dysfunction: role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol 282: H2316–H2323, 2002. [DOI] [PubMed] [Google Scholar]
- 110.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94: 1543–1553, 2004. [DOI] [PubMed] [Google Scholar]
- 111.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol 164: 3476–3479, 2000. [DOI] [PubMed] [Google Scholar]
- 112.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353–364, 2007. [DOI] [PubMed] [Google Scholar]
- 113.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164: 558–561, 2000. [DOI] [PubMed] [Google Scholar]
- 114.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF 3rd. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276: 10229–10233, 2001. [DOI] [PubMed] [Google Scholar]
- 115.Okubo S, Wildner O, Shah MR, Chelliah JC, Hess ML, Kukreja RC. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation 103: 877–881, 2001. [DOI] [PubMed] [Google Scholar]
- 116.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol 28: 2005–2016, 1996. [DOI] [PubMed] [Google Scholar]
- 117.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet 367: 356–367, 2006. [DOI] [PubMed] [Google Scholar]
- 118.Oyama J, Blais C Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789, 2004. [DOI] [PubMed] [Google Scholar]
- 119.Piper HM, Meuter K, Schafer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg 75: S644–S648, 2003. [DOI] [PubMed] [Google Scholar]
- 120.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998. [DOI] [PubMed] [Google Scholar]
- 121.Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, Billiar TR. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg 202: 407–417, 2006. [DOI] [PubMed] [Google Scholar]
- 122.Quinones MJ, Leor J, Kloner RA, Ito M, Patterson M, Witke WF, Kedes L. Avoidance of immune response prolongs expression of genes delivered to the adult rat myocardium by replication-defective adenovirus. Circulation 94: 1394–1401, 1996. [DOI] [PubMed] [Google Scholar]
- 123.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189: 615–625, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qureshi ST, Zhang X, Aberg E, Bousette N, Giaid A, Shan P, Medzhitov RM, Lee PJ. Inducible activation of TLR4 confers resistance to hyperoxia-induced pulmonary apoptosis. J Immunol 176: 4950–4958, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Riad A, Jager S, Sobirey M, Escher F, Yaulema-Riss A, Westermann D, Karatas A, Heimesaat MM, Bereswill S, Dragun D, Pauschinger M, Schultheiss HP, Tschope C. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J Immunol 180: 6954–6961, 2008. [DOI] [PubMed] [Google Scholar]
- 126.Roberts R, DeMello V, Sobel BE. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation 53: I204–I206, 1976. [PubMed] [Google Scholar]
- 127.Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, Schreurs BW, van den Berg WB, Radstake TR. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 176: 7021–7027, 2006. [DOI] [PubMed] [Google Scholar]
- 128.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation 67: 1016–1023, 1983. [DOI] [PubMed] [Google Scholar]
- 129.Rossig L, Fichtlscherer B, Breitschopf K, Haendeler J, Zeiher AM, Mulsch A, Dimmeler S. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem 274: 6823–6826, 1999. [DOI] [PubMed] [Google Scholar]
- 130.Sam F, Sawyer DB, Chang DL, Eberli FR, Ngoy S, Jain M, Amin J, Apstein CS, Colucci WS. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am J Physiol Heart Circ Physiol 279: H422–H428, 2000. [DOI] [PubMed] [Google Scholar]
- 131.Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki LM. Apoptosis in human acute myocardial infarction. Circulation 95: 320–323, 1997. [DOI] [PubMed] [Google Scholar]
- 132.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol 165: 7096–7101, 2000. [DOI] [PubMed] [Google Scholar]
- 133.Shappell SB, Taylor AA, Hughes H, Mitchell JR, Anderson DC, Smith CW. Comparison of antioxidant and nonantioxidant lipoxygenase inhibitors on neutrophil function. Implications for pathogenesis of myocardial reperfusion injury. J Pharmacol Exp Ther 252: 531–538, 1990. [PubMed] [Google Scholar]
- 134.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation 114: I270–I274, 2006. [DOI] [PubMed] [Google Scholar]
- 135.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189: 1777–1782, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I. Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation 108: 2905–2910, 2003. [DOI] [PubMed] [Google Scholar]
- 137.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 167: 2887–2894, 2001. [DOI] [PubMed] [Google Scholar]
- 138.Song W, Furman BL, Parratt JR. Delayed protection against ischaemia-induced ventricular arrhythmias and infarct size limitation by the prior administration of Escherichia coli endotoxin. Br J Pharmacol 118: 2157–2163, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem 69: 217–245, 2000. [DOI] [PubMed] [Google Scholar]
- 140.Takaesu G, Ninomiya-Tsuji J, Kishida S, Li X, Stark GR, Matsumoto K. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol Cell Biol 21: 2475–2484, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 21: 335–376, 2003. [DOI] [PubMed] [Google Scholar]
- 142.Tanaka M, Brooks SE, Richard VJ, FitzHarris GP, Stoler RC, Jennings RB, Arfors KE, Reimer KA. Effect of anti-CD18 antibody on myocardial neutrophil accumulation and infarct size after ischemia and reperfusion in dogs. Circulation 87: 526–535, 1993. [DOI] [PubMed] [Google Scholar]
- 143.Tanaka M, Ito H, Adachi S, Akimoto H, Nishikawa T, Kasajima T, Marumo F, Hiroe M. Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ Res 75: 426–433, 1994. [DOI] [PubMed] [Google Scholar]
- 144.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA 104: 13798–13803, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res 95: 700–707, 2004. [DOI] [PubMed] [Google Scholar]
- 146.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195: 99–111, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thornberry NA Caspases: key mediators of apoptosis. Chem Biol 5: R97–R103, 1998. [DOI] [PubMed] [Google Scholar]
- 148.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 281: 1312–1316, 1998. [DOI] [PubMed] [Google Scholar]
- 149.Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, Doevendans PA, van Echteld CJ, Joles JA, Quax PH, Piek JJ, Pasterkamp G, de Kleijn DP. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res 102: 257–264, 2008. [DOI] [PubMed] [Google Scholar]
- 150.Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem 276: 31332–31339, 2001. [DOI] [PubMed] [Google Scholar]
- 151.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem 277: 15107–15112, 2002. [DOI] [PubMed] [Google Scholar]
- 152.Vinten-Johansen J Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 61: 481–497, 2004. [DOI] [PubMed] [Google Scholar]
- 153.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412: 346–351, 2001. [DOI] [PubMed] [Google Scholar]
- 154.Wang Y, Guo Y, Zhang SX, Wu WJ, Wang J, Bao W, Bolli R. Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J Mol Cell Cardiol 34: 5–15, 2002. [DOI] [PubMed] [Google Scholar]
- 155.Wang YP, Sato C, Mizoguchi K, Yamashita Y, Oe M, Maeta H. Lipopolysaccharide triggers late preconditioning against myocardial infarction via inducible nitric oxide synthase. Cardiovasc Res 56: 33–42, 2002. [DOI] [PubMed] [Google Scholar]
- 156.Ward C, Murray J, Clugston A, Dransfield I, Haslett C, Rossi AG. Interleukin-10 inhibits lipopolysaccharide-induced survival and extracellular signal-regulated kinase activation in human neutrophils. Eur J Immunol 35: 2728–2737, 2005. [DOI] [PubMed] [Google Scholar]
- 157.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest 111: 1497–1504, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7: 837–847, 1997. [DOI] [PubMed] [Google Scholar]
- 159.Wollert KC, Heineke J, Westermann J, Ludde M, Fiedler B, Zierhut W, Laurent D, Bauer MK, Schulze-Osthoff K, Drexler H. The cardiac Fas (APO-1/CD95) receptor/Fas ligand system: relation to diastolic wall stress in volume-overload hypertrophy in vivo and activation of the transcription factor AP-1 in cardiac myocytes. Circulation 101: 1172–1178, 2000. [DOI] [PubMed] [Google Scholar]
- 160.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431–1433, 1990. [DOI] [PubMed] [Google Scholar]
- 161.Wright SD, Tobias PS, Ulevitch RJ, Ramos RA. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med 170: 1231–1241, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301: 640–643, 2003. [DOI] [PubMed] [Google Scholar]
- 163.Yaoita H, Ogawa K, Maehara K, Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 97: 276–281, 1998. [DOI] [PubMed] [Google Scholar]
- 164.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. [DOI] [PubMed] [Google Scholar]
- 165.Yuan J Transducing signals of life and death. Curr Opin Cell Biol 9: 247–251, 1997. [DOI] [PubMed] [Google Scholar]
- 166.Zacharowski K, Frank S, Otto M, Chatterjee PK, Cuzzocrea S, Hafner G, Pfeilschifter J, Thiemermann C. Lipoteichoic acid induces delayed protection in the rat heart: a comparison with endotoxin. Arterioscler Thromb Vasc Biol 20: 1521–1528, 2000. [DOI] [PubMed] [Google Scholar]
- 167.Zacharowski K, Otto M, Hafner G, Chatterjee PK, Thiemermann C. Endotoxin induces a second window of protection in the rat heart as determined by using p-nitro-blue tetrazolium staining, cardiac troponin T release, and histology. Arterioscler Thromb Vasc Biol 19: 2276–2280, 1999. [DOI] [PubMed] [Google Scholar]
- 168.Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, Lee PJ. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol 175: 4834–4838, 2005. [DOI] [PubMed] [Google Scholar]
- 169.Zhu X, Bagchi A, Zhao H, Kirschning CJ, Hajjar RJ, Chao W, Hellman J, Schmidt U. Toll-like receptor 2 activation by bacterial peptidoglycan-associated lipoprotein activates cardiomyocyte inflammation and contractile dysfunction. Crit Care Med 35: 886–892, 2007. [DOI] [PubMed] [Google Scholar]
- 170.Zhu X, Zhao H, Graveline AR, Buys ES, Schmidt U, Bloch KD, Rosenzweig A, Chao W. MyD88 and NOS2 are essential for toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol 291: H1900–H1909, 2006. [DOI] [PubMed] [Google Scholar]