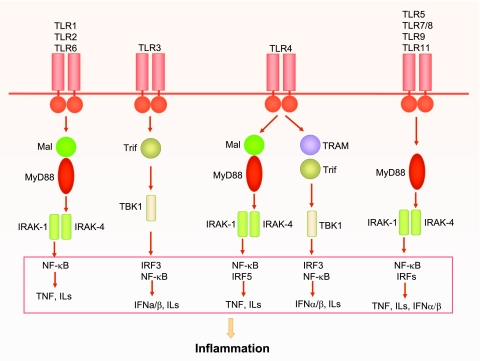
Fig. 1.
Toll-like receptor (TLR) signaling. All TLRs are transmembrane proteins with a large extra-cellular domain containing leucine-rich repeats and a unique cytoplasmic Toll/IL-1 receptor (TIR) domain. TLRs exist in dimmers, and all TLR family members, except TLR3, signal through the key adaptor myeloid differentiation primary-response gene 88 (MyD88) to recruit downstream interleukin (IL)-1 receptor-associated kinases (IRAKs). In some TLR signaling, such as TLR2 and TLR4, MyD88 adaptor-like protein (Mal) is required for recruiting MyD88 to their receptors, whereas in others, such as TLR5, TLR7, TLR9, and TLR11, Mal is not required. TLR1 and TLR2 or TLR2 and TLR6 form heterodimers that signal through Mal/MyD88. TLR3 signals through the adaptor TIR-domain-containing adaptor protein inducing interferon-β (IFN-β)-mediated transcription-factor (Trif), which recruits and activates TNF receptor-associated factor-family member-associated NF-κB activator-binding kinase 1 (TBK1). In addition to Mal/MyD88-dependent pathway, TLR4 can also signal through a MyD88-independent pathway that activates TBK1 via Trif-related adaptor molecule (TRAM)-Trif-dependent mechanism. TLR5, TLR7/8, TLR9, and TLR11 use only MyD88 as its signaling adaptor. These kinases ultimately activate transcription factors such as nuclear factor-κB (NF-κB) and IFN regulatory factor (IRFs), which result in production of various proinflammatory cytokines such as tumor necrosis factor (TNF), ILs, and IFNs.