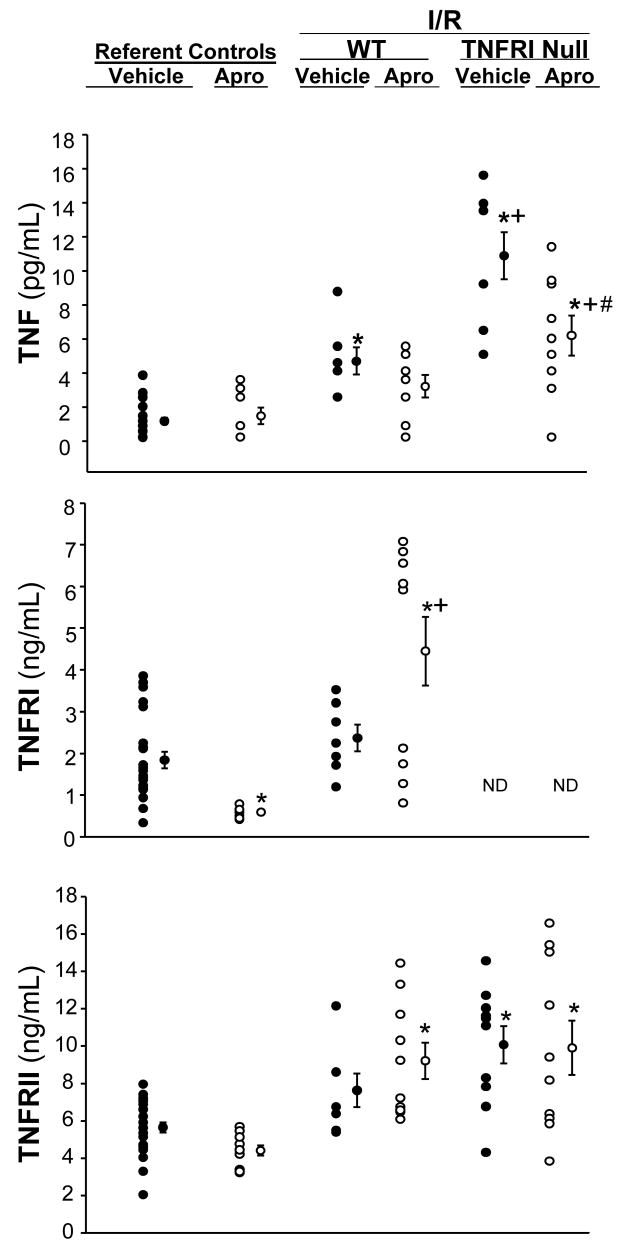
Figure 3.
Plasma tumor necrosis factor-α (TNF), TNF receptor I (TNFRI) and TNFRII were computed in referent wild type (WT) control mice (maintained under equivalent conditions without I/R) receiving vehicle or aprotinin (APRO), in WT mice following I/R with or without aprotinin, and in TNFRInull mice following I/R with or without aprotinin. In both WT and TNFRInull mice following I/R, plasma TNF levels increased, with a more robust increase observed in the TNFRInull mice. Aprotinin administration prior to I/R blunted TNF release. TNFRI levels were reduced with aprotinin administration only, in the absence of I/R. However, TNFRI levels significantly increased with aprotinin administration following I/R. As expected, TNFRI levels were not detectable in the TNFRInull mice. Plasma TNFRII levels were significantly elevated across all treatment groups as compared to referent vehicle controls. (*p<0.05 vs referent vehicle controls; +p<0.05 vs respective WT values, #p<0.05 vs respective vehicle values)