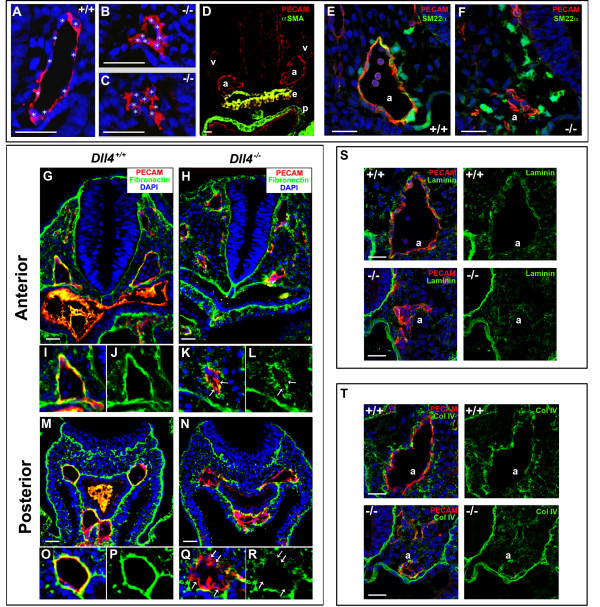
Figure 2.
The Dll4-/- arterial endothelial cells have an abnormal morphology, become more aggregated and display a defective basement membrane. (A-C) Anti-PECAM-1 staining of 8 ss embryo cryosections showing the morphology of Dll4+/+ and Dll4-/- dorsal aortae endothelial cells. The white stars label the DAPI stained endothelial nuclei. Dll4-/- endothelial cells are more aggregated and fail to develop a stretched spindle-like form which leads to a reduced (B) or absent aortic lumen (C). (D) Anti-PECAM-1 (red) and anti-αSMA (green) staining of 16 ss cryosection of a Dll4+/+ embryo showing the absence of arterial (a) and venous (v) endothelium associated smooth muscle cells. Note the strong αSMA staining in the endoderm (e) and pericardium (p). (E, F) Both control and mutant dorsal aortae (a) are surrounded by neural crest derived smooth muscle cell progenitors with high amounts of Sm22α protein (in green). (G-R) Anti-PECAM-1 (red), anti-fibronectin (green) and DAPI (blue) staining of cryosections from the anterior (G-L) and posterior (M-R) region of 11 ss Dll4+/+ and Dll4-/- embryos. Figures I-L and O-R are close ups of the upper left dorsal aortae and show the defective basement membrane (green) in both the anterior and posterior regions of the Dll4-/- aortae. In the mutants many endothelial cells are not in direct contact with the underlying, fibronectin-rich, extracelular matrix (arrows). (S, T) At this stage of development the aortic (a) basement membrane is relatively poor in laminin (S, control) and collagenIV (T, control) when compared with fibronectin (J and P). The reduced Dll4-/- dorsal aortae have even less and more irregular laminin and collagenIV coverage. (Scale Bars: 30 μm).