Figure 4.
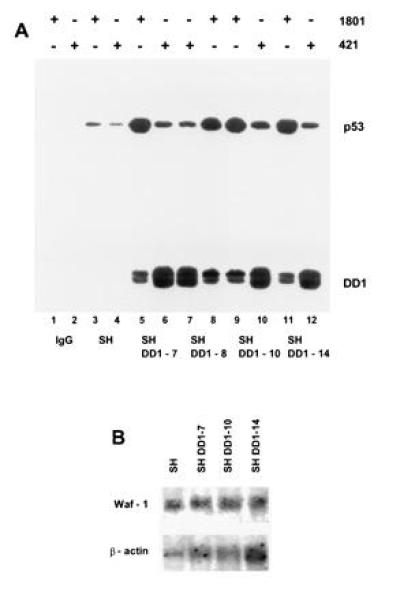
wt p53 is shuttled into the nucleus by means of heterocomplex formation with C-terminal peptide, a dominant negative mutant. (A) Coimmunoprecipitation of whole cell extracts (1.5 mg of total protein, lanes 3–12) of SH cells (lanes 3 and 4) and sublines DD1-7 (lanes 5 and 6), DD1-8 (lanes 7 and 8), DD1-10 (lanes 9 and 10), and DD1-14 (lanes 11 and 12) with either PAb 1801 (1.5 μg) (lanes 1, 3, 5, 8, 9, and 11) or PAb 421 (1.5 μg) (lanes 2, 4, 6, 7, 10, and 12) followed by immunoblotting with rabbit CM-1 antiserum. Control lanes 1 and 2 lack cell extracts. Note that PAb 421 regains partial epitope recognition after sonication of SH cell lysates (compare Fig. 2A with Fig. 3A and with Fig 4, lane 4. (B) Waf-1 mRNA analysis (10 μg per lane) comparing SH parental and several DD1 lines. Despite high levels of nuclear wt p53, no induction of p53 response genes is seen. The blot was stripped and reprobed with human β-actin cDNA as loading control.
